- About us
- Asset Management
- Home
- Interpersonal relation
- Points store
- Production and operation
- 01, Production systems and operations management
- 02, Operations Strategy
- 03, Tours of operations
- 04, Forecasting
- 05, Product Design and Operations
- 06, Capability Planning and Facility Location
- 07, Selecting the Process Structure and Technology
- 08, Process Design and Facility Layout
- 09, Waiting or Queueing System
- 10, Job design, Work methods, and Organization
- 11, The Quality management system
- Quality Management Doctor
- Aseptic production of milk and juice
- 00.Introduction
- 01.UHT processing
- 02.Aseptic packaging
- 03. Sterilising Effect
- 04. H2O2 as a sterilant
- 05. Packaging material
- 06. Application of microbiology to UHT processing and aseptic packaging
- 07. Installation
- 08. Cleaning and house keeping
- 09. Commissioning
- 10. Changes during milk processing
- 11. Product changes during storage
- 12. Quality control of aseptic product-Part1
- 12. Quality control of aseptic product-Part2
- Bacteria, virus an human life
- Food Safety and Microbial Quality
- Keeping Cows Cool and Comfortable to Improve milk production of cow
- Operation and quality management
- Aseptic production of milk and juice
- Quality Management Doctor
- OCE to measure the personnel performance?
- TPM world class management
- Team building
022. Sterilisation of the Packaging Material
English
2.Sterilisation of the Packaging Material
In aseptic packaging procedures, sterilisation of the packaging material (food contact surface) is achieved with few exceptions by chemical means (61). By far the most common chemical used for this purpose is hydrogen peroxide (H2O2) (93, 142). Of importance are the microbiological efficiency of the sterilisation process and the elimination of the chemical which might get into the filled product as a residue.
Depending on the make of the aseptic packaging equipment, different means of applying the sterilant are used: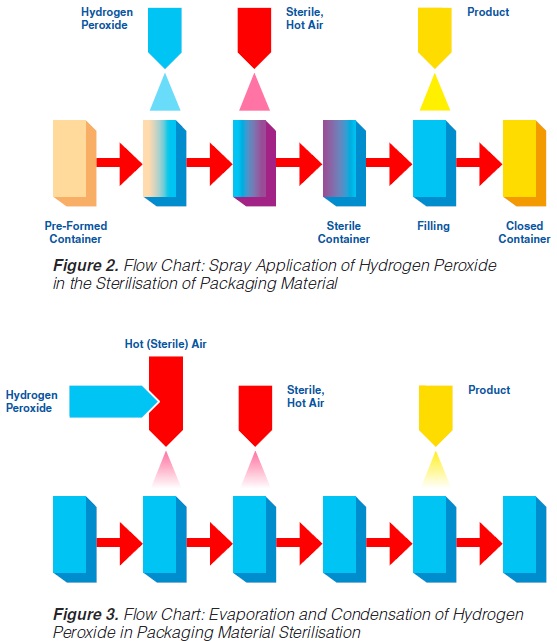
1) spray;
2) vapour;
3) roller systems;
4) immersion bath, etc.
2.1 Spray Application
The spraying (“fogging”) of hydrogen peroxide is used in some aseptic packaging systems which operate intermittently and use pre-formed blanks (61, 74, 93, 171).
A certain amount of H2O2 is sprayed into each pre-erected container through a spray nozzle (figure 2). For proper function (sterilisation), the food contact surface of the container needs to be covered completely with the spray solution. Because of the hydrophobic characteristics of plastic materials in general and polyethylene in particular, it has been found (91, 145) that only 20-30% of the carton surfaces are wetted. In spite of this, good killing effects were achieved: up to 6 log reductions when tested with Bacillus subtilis spores have been reported (145), probably because of the subsequent evaporation.
Hot sterile air is blown into the container to attain the temperature necessary for the sterilisation process and to remove the H2O2 from the food contact surface. Using sterile air at
180°C for drying, 5 to 7 decimal reductions of Bacillus subtilis spores were attained (91, 113).
The killing efficiency of low concentrations (~ 0.5% or less) of hydrogen peroxide was greatly enhanced by the simultaneous use of ultraviolet radiation (44, 45).
2.2 Application by Vapour
In systems applying hydrogen peroxide by vaporisation, liquid H2O2 is injected into a stream of hot (sterile) air, then vaporised and condensed on the surfaces to be sterilised (93). A better coverage
of surfaces is obtained since the condensing droplets are smaller. Subsequently, the hydrogen peroxide is heated and evaporated by blowing hot, sterile air into the containers (figure 3).
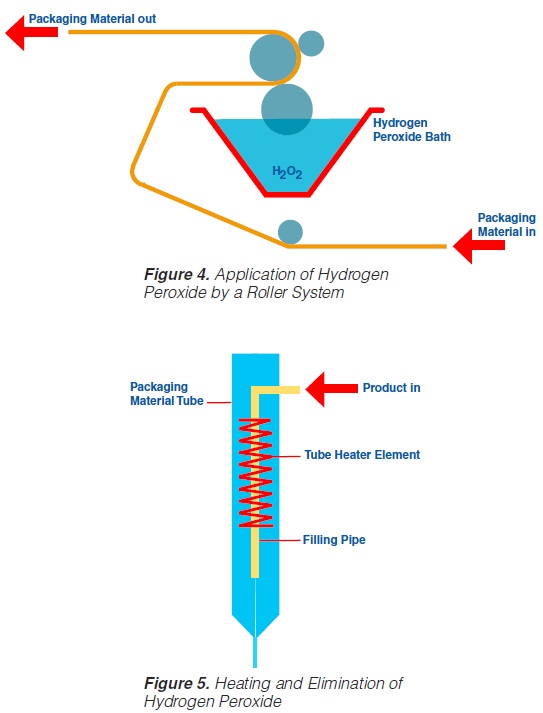
2.3 Application by Roller System
Roller systems (figure 4) permit the application of liquid hydrogen peroxide on to flat food contact surfaces (59, 60, 61, 74, 93). Packaging material sterilisation thus becomes possible before the containers are formed (figure 4).
In order to obtain a film of the water like H2O2 liquid covering the total food contact surface, a wetting agent – about 0.2 to 0.3% of PSM, (polyoxyethylenesorbitan-
monolaurate), or equivalent is recommended - has to be added to the hydrogen peroxide. After application of the sterilant on to the food contact surface, the packaging material web is formed into a tube and sealed longitudinally. The actual sterilisation requires the hydrogen peroxide covering the
packaging material food contact surface to be at a high temperature. An electrical element (“tube heater”) provides the temperature necessary (105-110°C) for the sterilisation process and, simultaneously, eliminates the H2O2 (figure 5).
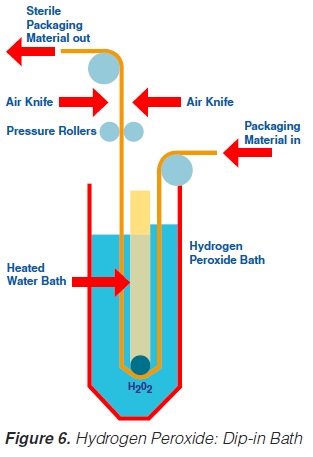
2.4 Use of an Immersion Bath
Likewise, the use of a hydrogen peroxide bath permits sterilisation of a plane packaging material web (59, 61, 69, 73, 74, 93, 171) prior to the actual forming of the container. The packaging material is sterilised by its passage through liquid hydrogen peroxide. The temperature necessary for the sterilisation process is obtained by heating the hydrogen peroxide solution indirectly by means of a water bath which is placed inside the H2O2 bath (figure 6). A concentration of 30%, a temperature of 70°C and an exposure time of about 10 seconds are necessary to achieve sufficient killing of bacterial endospores (74). In some models, the hydrogen peroxide is removed from the packaging material:
a) by a pair of pressure rollers that squeeze the excess chemical back into the hydrogen peroxide bath; and
b) by a pair of air knives that blow hot, sterile air on to both sides of the packaging material web.
After passage through the bath and removal of the hydrogen peroxide, the sterile, flat packaging material web is formed into a tube and sealed longitudinally.
