11, The Quality management system
11.1 Quality philosophy
Companies perceived as producing products of poor quality have either failed or are struggling for survival, while those recognized for quality have generally flourished. During 1970s and 1980s, product defect rate of Japanese was 10-100 times lower than his competitors. Now US and Europe have eliminated this gap with the quality level increasing from 3 sigma (AQL is 3/1000) to 6 sigma (AQL is 2 per million) in electron industry. The consumers who buy products with good quality are more loyal than those who buy based on price and always change to competitors' products with lower price. We can also find that producers with good quality have lower cost because of using advanced management method to avoid quality loss caused cost. The approaches of Deming, Juran, Shingo and others actually support each other producing total quality management (TQM) which deploys quality management in all crossed departments but not only quality department, in every area of process but not the end products and by every personnel but not only quality people.
11.2 What is quality
Quality is the competence of product or service to satisfy the customer's need or expectation. In other words, quality is customer oriented with promising something to consumer. Specific quality dimensions varies in different products with the general references as the followings.
1, Performance. Performance is specificication promisiing to and recognized by customer such as density, viscosity and volume etc.
2, Features. Such as attracting desining of the packing, high resolution screen of cell phone etc.
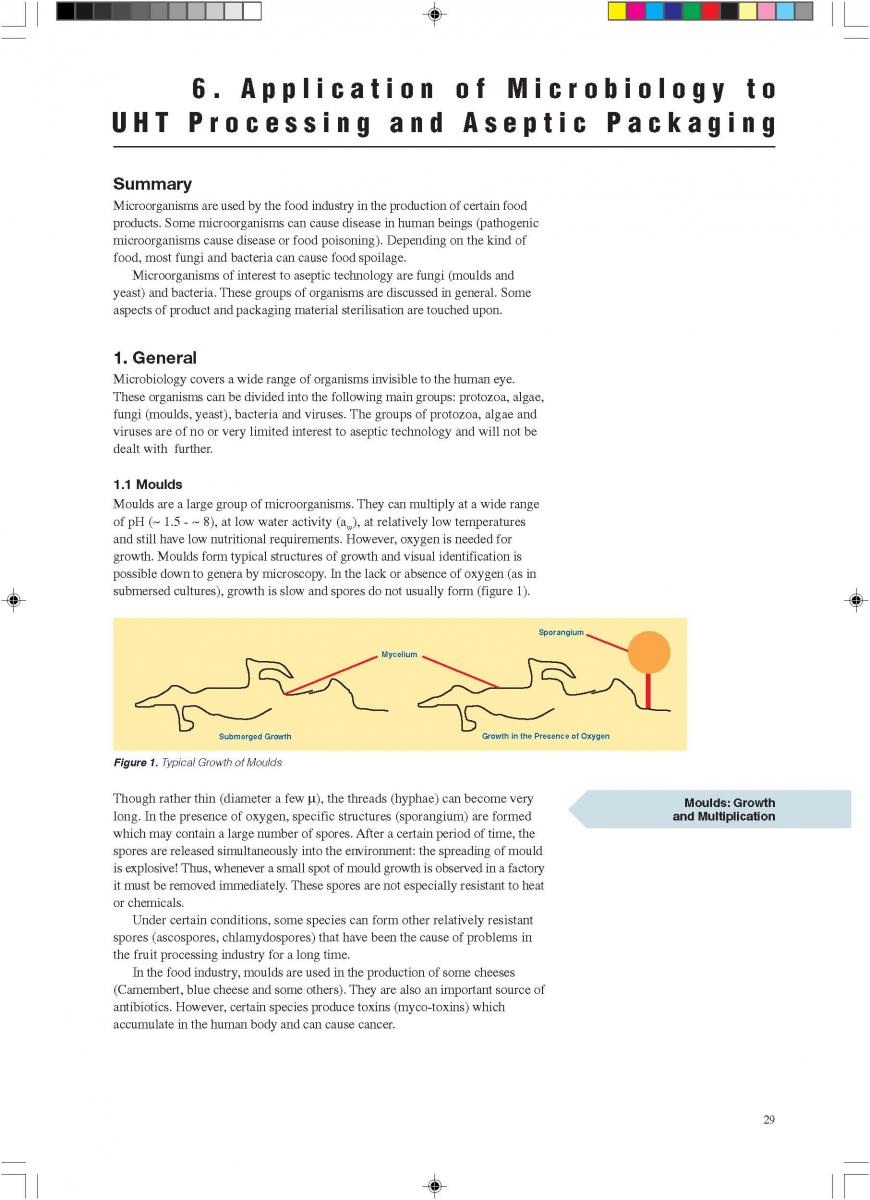
3, Reliability and durability. Reliability is the quality consistency under normal maintenance, production and consuming environment such as in normal living environment, one type of TV can always displays the expected pictures. And durability measures the product life etc.
4, Maintainability and serviceability. Maintainability measures whether one product such as milk filler can be mainatined to keep its quality and serviceability measures whether there is after sales service such as on-site support or remote telephone hot line assistant support.
5, Sensory charicteristics. Products with high nutrition without being tasty or good smell are generally not good quality products.
6, Ethical profile and image. Different people have different believing affecting their judge or acception of some specific products.
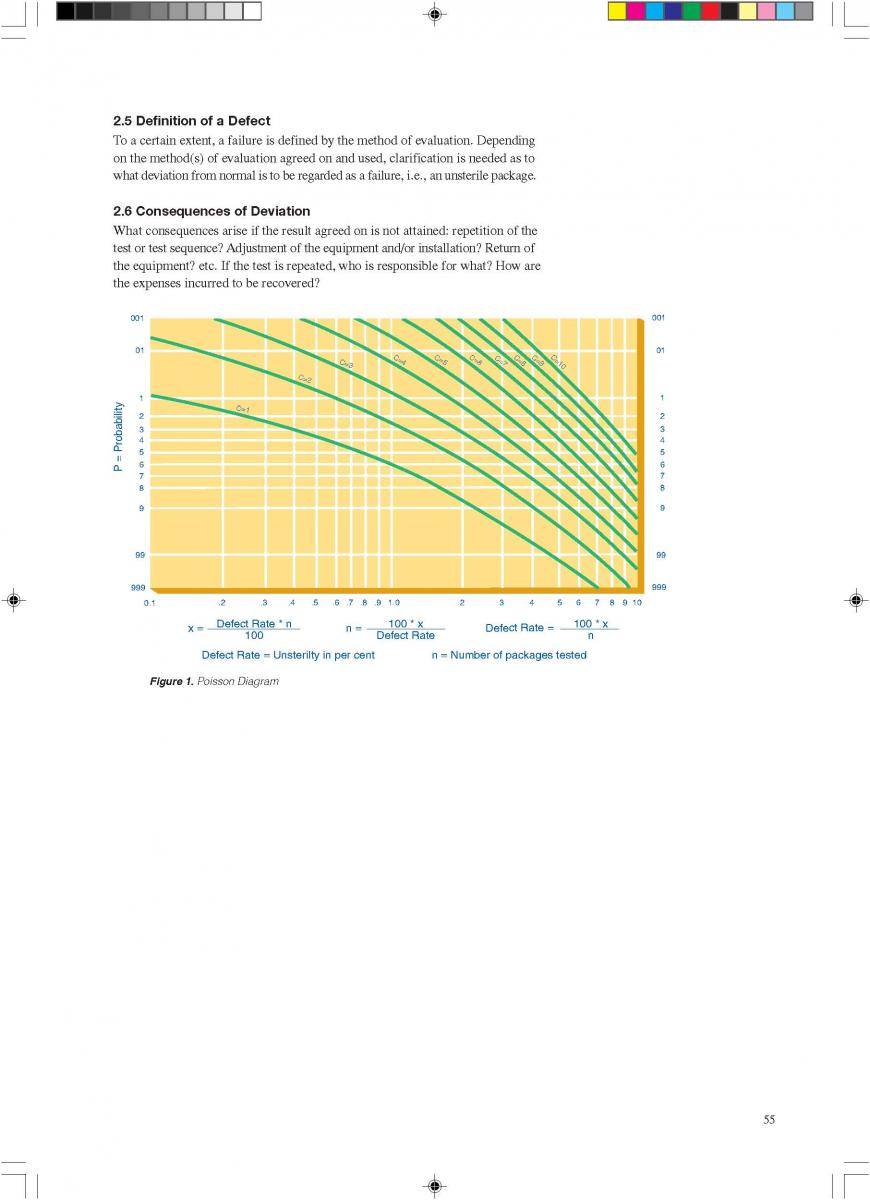
11.3 Quality cost audit
We need to know wether it is worth to produce one specific level of quality. In other words, we need to find the equivalent point of quality input and output. Generally, we need to consider failure cost, inspection cost and prevention cost such as quality control. Traditionally, the cost distribution of failure, inpection and prevention are 50-80%, 15-40% and 5-10%. After TQM, the cost distribution of failure, inpection and prevention are 20-50%, 15-40% and 25-50%.
Quality Management Doctor
 Companies perceived as producing products of poor quality have either failed or are struggling for survival, while those recognized for quality have generally flourished. The consumers who buy products with good quality are more loyal than those who buy based on price and always change to competitors' products with lower price. We can also find that producers with good quality have lower cost because of using advanced management method to avoid quality loss caused cost. The approaches of Deming, Juran, Shingo and others actually support each other producing total quality management (TQM) which deploys quality management in all crossed departments but not only quality department, in every area of process but not the end products and by every personnel but not only quality people.
Companies perceived as producing products of poor quality have either failed or are struggling for survival, while those recognized for quality have generally flourished. The consumers who buy products with good quality are more loyal than those who buy based on price and always change to competitors' products with lower price. We can also find that producers with good quality have lower cost because of using advanced management method to avoid quality loss caused cost. The approaches of Deming, Juran, Shingo and others actually support each other producing total quality management (TQM) which deploys quality management in all crossed departments but not only quality department, in every area of process but not the end products and by every personnel but not only quality people.
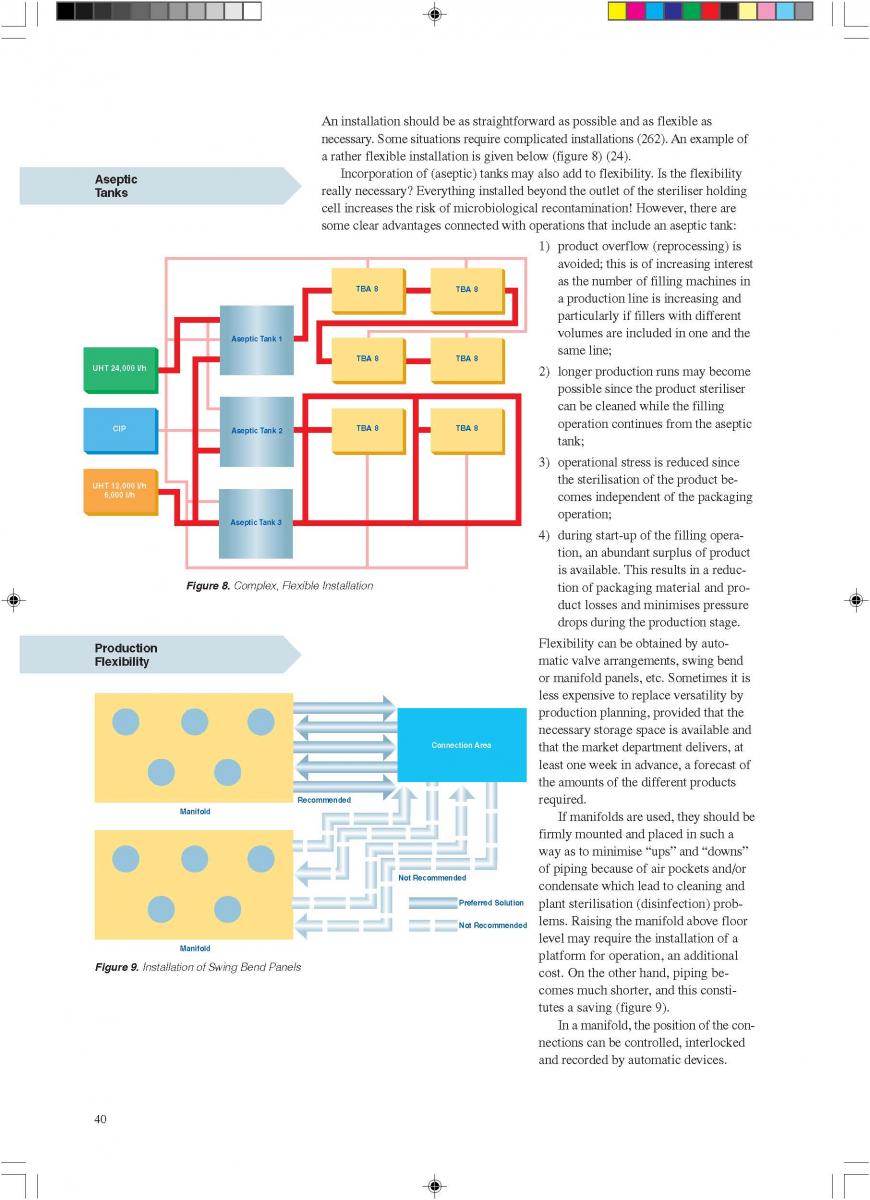
Aseptic production of milk and juice
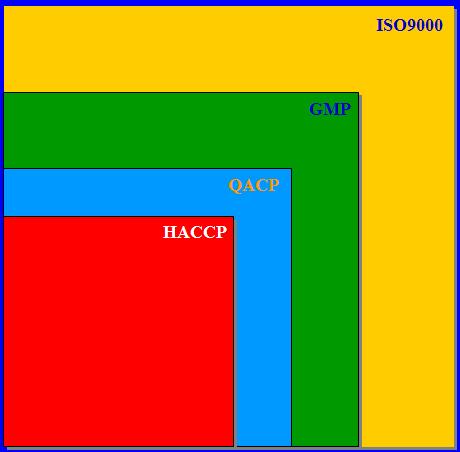
The intention of this book is to present some of the knowledgeacquired during many years of practical work on the qualitycontrol and quality assurance of long-life products. Differentfactors have an impact on product quality – the standard of the rawmaterials, the equipment involved, the processes applied, opera-tional procedures, installation, and so on. In order to produce ahigh quality product, a thorough understanding of these factors isnecessary. An attempt has been made to discuss and present thematerial in a comprehensible way concentrating on microbio-logical aspects. The HACCP (QACP) concept has been used as amodel for process control.UHT-treated and aseptically packaged products have beenproduced in large quantities for quite some time. So far, the litera-ture available concentrates on particular aspects of product andequipment characteristics and the technology involved. A moregeneral presentation appears to be lacking. Hopefully, our workwill help close the gap.To a large extent, the available “old” literature has beenconsulted. The new literature is readily accessible through dataprofile searches. In addition, more than 25 years of practical fieldexperience has gone into this study.The different topics are arranged in such a way that each chap-ter stands as an independent entity. Of necessity, this has led tosome repetitions. We hope that the reader will accept the problemand make allowances for the consequences. It is also our hope thatthis study will contribute to the production of long-life productswith a high level of quality.Finally, we would like to express our appreciation to all theTetra Pak people who have helped to prepare this book and whohave contributed with valuable comments on the contents. Thisapplies particularly to Gösta Odelberg B. Sc., Regional ManagerFiSQA (Field Service Quality Assurance) and Dr. Charles Sizer.Åkarp, August 1998
Dr. Bernhard von Bockelmann Dr. Irene von Bockelmann
You can use cash or points to buy this book at points store or related display area. English version and name is Quality management of aseptic production, Chinese version and name is 无菌生产质量管理.
00.Introduction
Commercially sterile products have been on the market for a long time. Direct
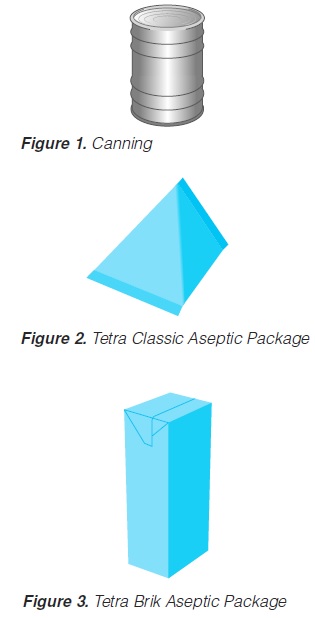
UHT (Ultra High Temperature) processing was introduced at the beginning ofthe 20th century. At that time, the technology was used only as a pre-treatmentfor products to be filled into cans for subsequent retorting. The first UHT-treatedand aseptically packaged products were in cans and were shown at an agricultural exhibition in London in the mid 1920s. The product (milk) was not a commercial success. During the early 1930s, aseptic canning was developed in the USA (69).
In 1961, aseptic packaging procedures were introduced (74, 93, 243) using flexible packaging material (a laminate of wax, paper and polyethylene): theTetra Pak system (“Tetra Classic Aseptic” or TCA system). The paperboardcarton and black colouring provided protection against the entry of light. How-ever, gas barrier characteristics were rather poor. In the meantime, UHT process-ing systems were in more general use. After initial problems, the combination ofUHT processing and aseptic packaging in the TCA system gained market success(“long-life products”).
In 1969, a brick-shaped package was introduced (“Tetra Brik Aseptic” or TBAsystem). The structure of the packaging material was more complex: a poly-ethylene-paper-polyethylene-aluminium foil-polyethylene laminate. Such amaterial provides protection against the entry of light and acts as an effective gasbarrier. The shape of the container offered considerable advantages in storage,transportation and handling. It also provided the real break through for UHTprocessing and aseptic packaging technology. Today, larger quantities of both low and high-acid, shelf-stable food products are processed by inflow (UHT)methods and subsequently packaged under aseptic conditions. Milk andmilk-based products were the first long-life food products and still remain thelargest commodity in terms of volume.
Processes that stabilise food thermally have been a major benefit to mankindby providing stability and safety in food. Although commercial canning isprobably the most reliable and safest method of food preservation, it is not perfect. In trying to improve the technology, the present low risk of contamina-tion present in simple straight forward technologies must sometimes be sacrificed to gain better quality and economy.
Aseptic technology is considered to have the followingadvantages over conventional technologies (254):
a)new package forms;
b)savings in energy and packaging costs;
c)convenience; and
d)improved product quality.
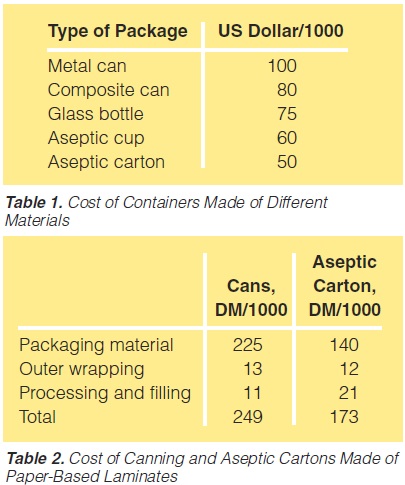
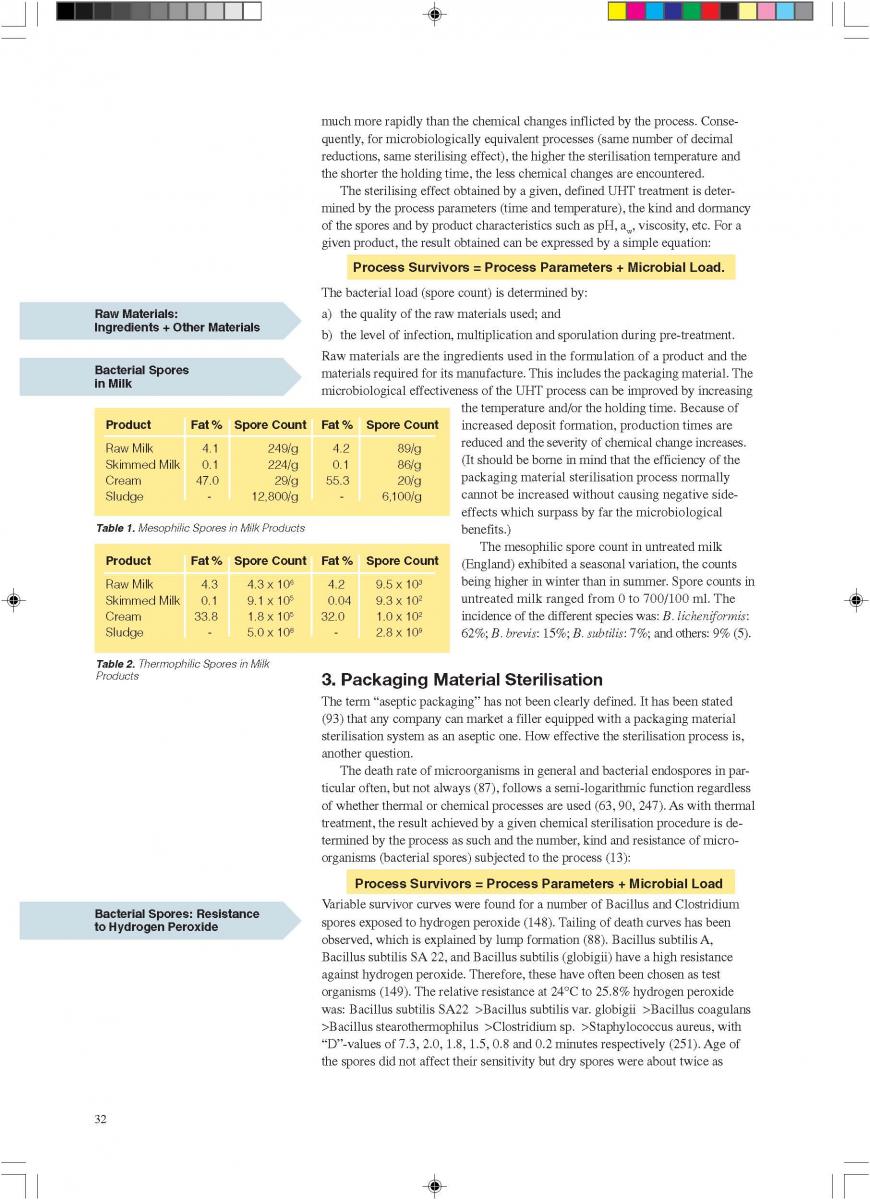
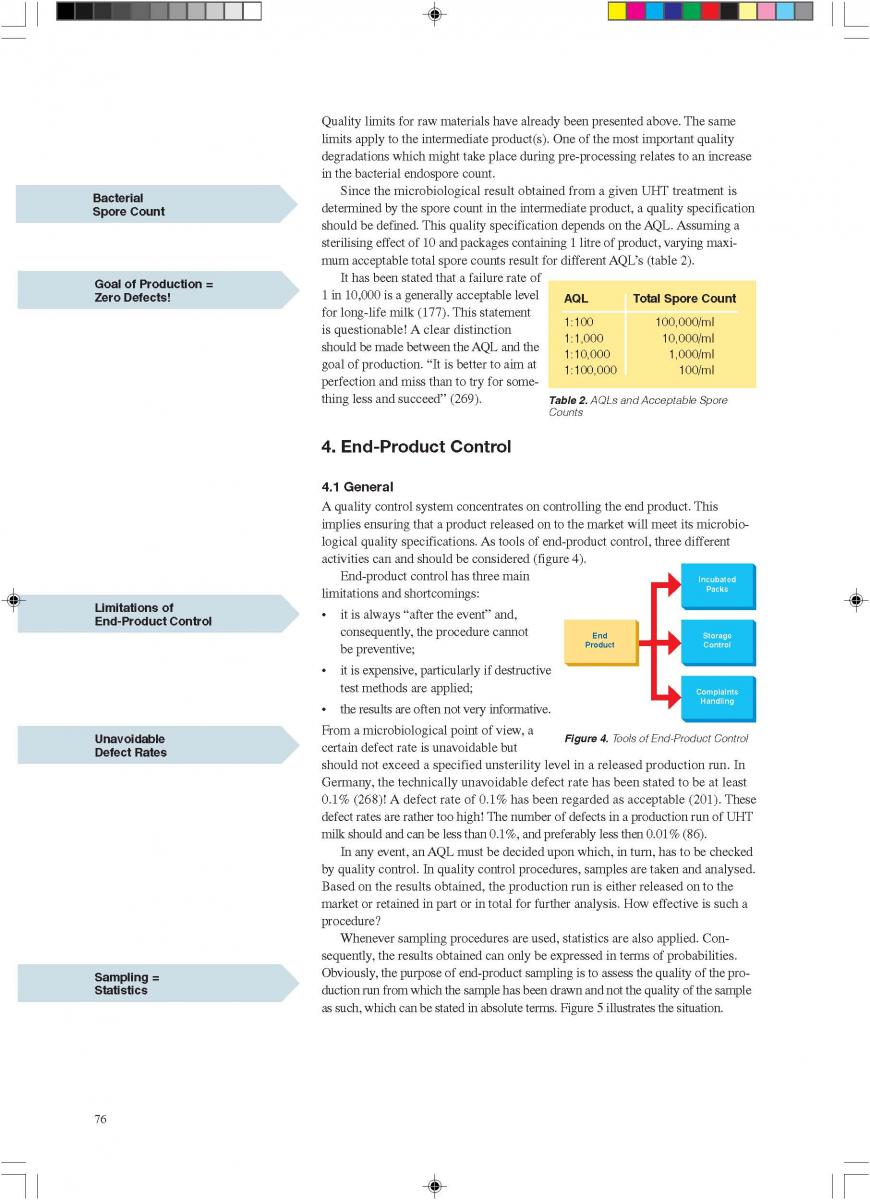
A comparison of the price of different packaging alternativesis shown in table 1 (278).In table 2, the total cost of canning is compared to theproduction of a long-life food product (table 2, 179).
01.UHT processing
Summary
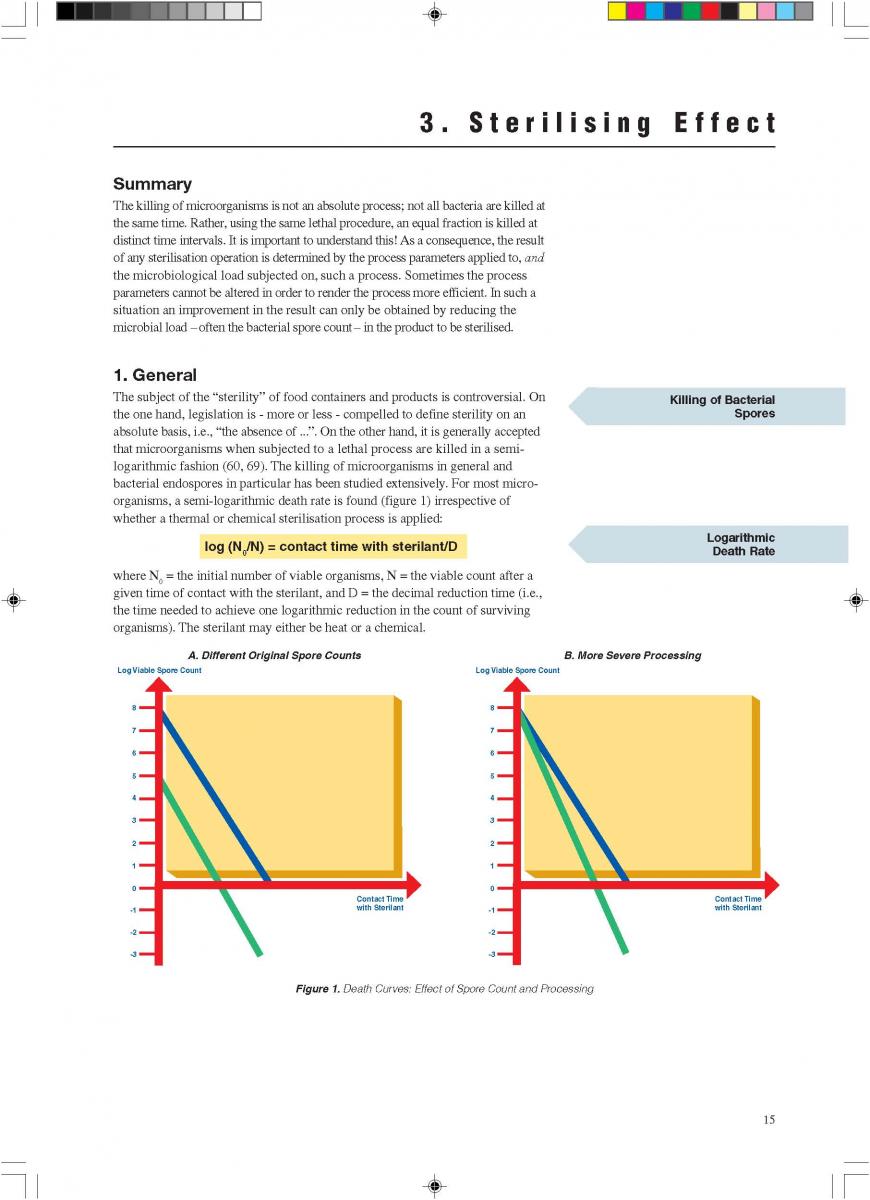
Food products are processed by UTH Methods in order to obtain a commercially sterile product. Rapid heating, short holding and rapid cooling minimise the occurrence of chemical change. A short description is given of the principles involved in indirect and direct heating systems.
011.General description
1. General
Ultra-high-temperature treatment is a continuous inflow
process. It is based on the rapid heating of the product to the sterilisation temperature and short holding at that temperature, followed by rapid cooling. The purpose of UHT treatment is to achieve commercial sterility of the product. Full sterilisation efficiency requires rapid heat transfer which is only possible in liquid systems. If powders are used in the formulation of a product to be UHT-treated, special attention has to be paid to proper soaking: all powder particles must be completely wetted through.
1.1 Low-Acid, Liquid-Food Products
Low-acid food products are characterised by a pH value of > 4.5 or > 4.6, depending on local food legislation. These products require careful treatment because:
a) microorganisms can grow and multiply. Bacterial spores are also able to germinate and cause product spoilage;
b) practically all pathogenic (disease-causing) microorganisms can develop.
Consumption of products thus spoiled may lead to food poisoning and/or food-borne infections.
Typical processing temperatures for low-acid foods are 130-150°C with holding times of a few seconds (60), usually 4 seconds.
1.2 High-Acid Liquid-Food Products
High-acid food products have a pH value equal to 4.5 or 4.6 or less. These are mainly fruit juices, fruit juice drinks and “belly washers”. High-acid food products are safer than low-acid foods because:
a) they are not prone to pathogenic bacteria and are therefore regarded as safe from the point of view of public health;
b) bacterial spores cannot germinate under high-acid conditions and, consequently, cannot cause food spoilage;
c) the sterilising efficiency of any heat treatment increases with decreasing pH. Thus, lower temperatures can be applied in order to achieve commercial sterility;
d) in addition, some organic acids common in fruits specifically decrease the temperature resistance of possible spoilage organisms;
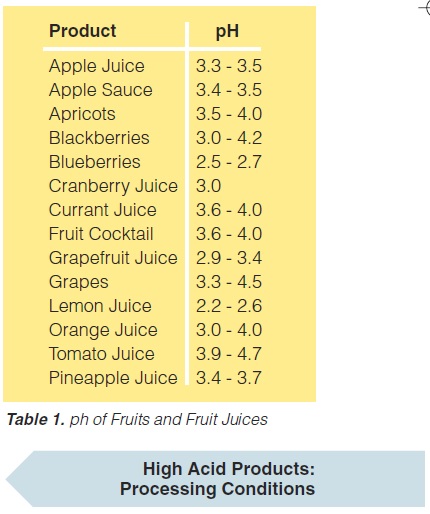
e) the main spoilage microorganisms are yeast, mould, and a few bacteria (Lactobacillus, Streptococcus, and some others). Processing temperatures for high-acid foods are rather low. With few exceptions temperatures of 85 -95°C with holding times of 30 to 15 seconds, sometimes a few minutes, are sufficient (60). However, there are some exceptions, particularly tomato products which may require considerably higher temperatures, often above 100°C. The pH values of a number of fruits and fruit juices (131) are given in table 1.
1.3 Acidified Liquid-Food Products
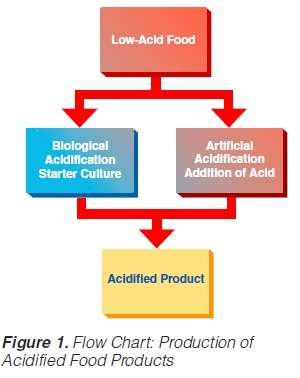
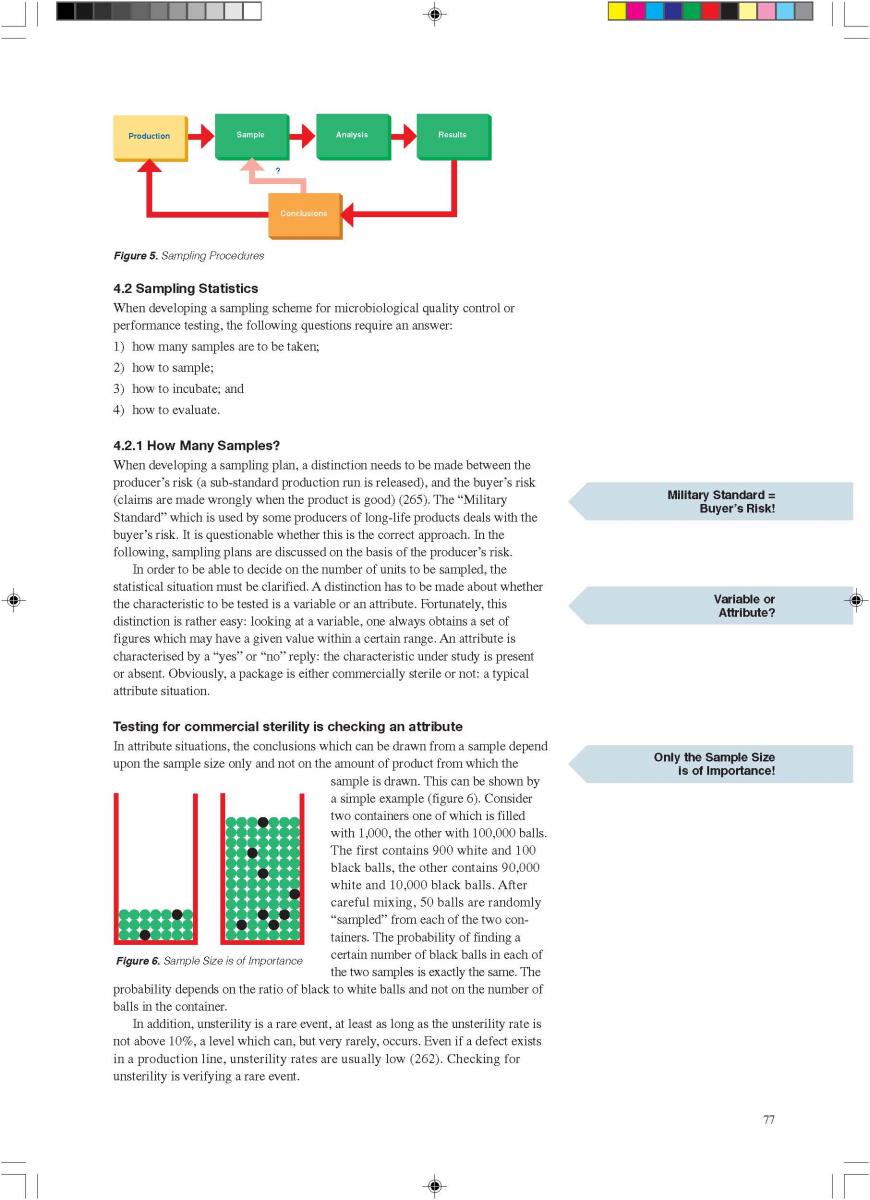
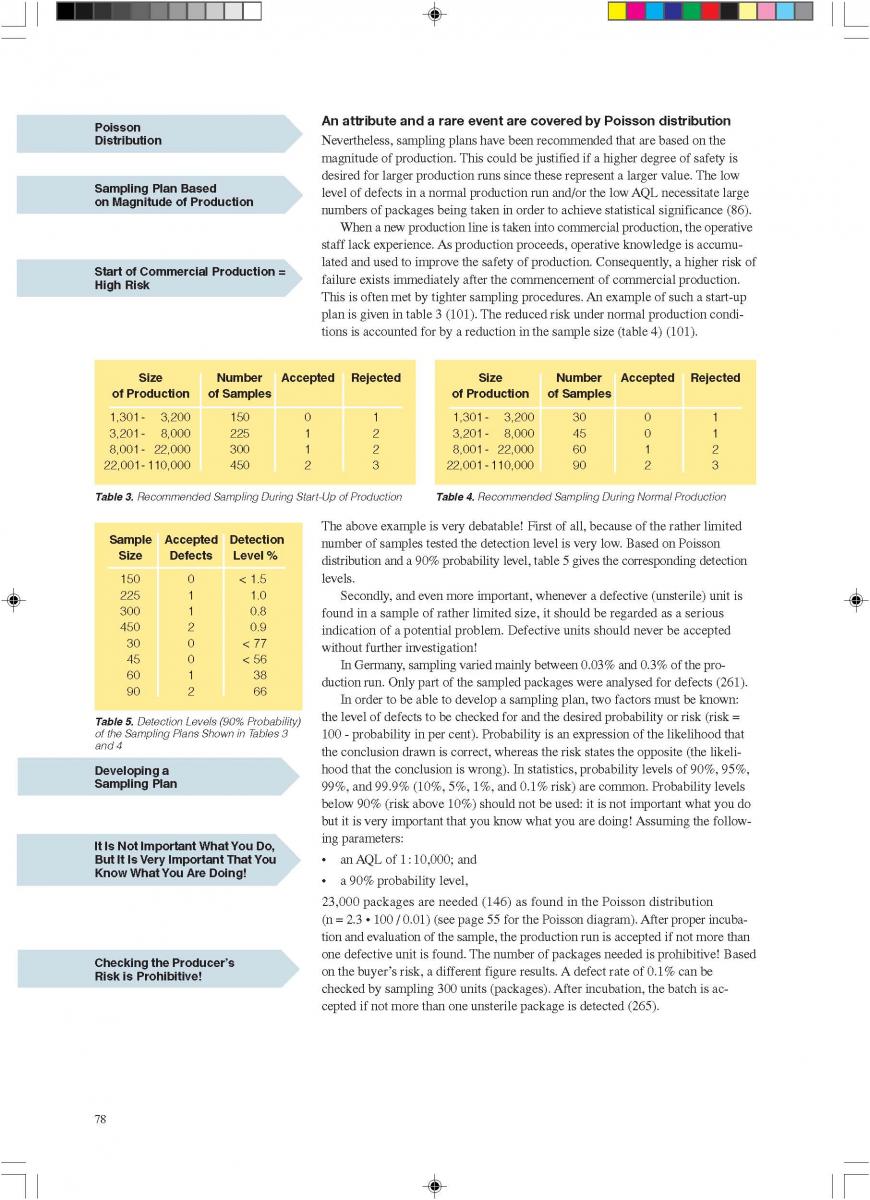
Acidified foods are low-acid products, the pH of which has been lowered into the high-acid range by pre-processing. Such products have the same microbiological advantages as high-acid food products. The acidification process is crucial. It is necessary to achieve an even and low pH throughout the product. Acidification can be carried out biologically by the addition and growth of a starter culture, usually Lactobacillus and/or Streptococcus followed by a ripening period at a suitable temperature. Another possibility is an artificial or chemical procedure in which an acid (usually citric or lactic acid) is added to the product which has to be mixed thoroughly afterwards (Figure 1). To achieve commercial sterility, the acidified product has to be processed after the acidification process, usually by heat treatment, and then packaged.
012.Indirect UHT
 2. Indirect UHT Systems
2. Indirect UHT Systems
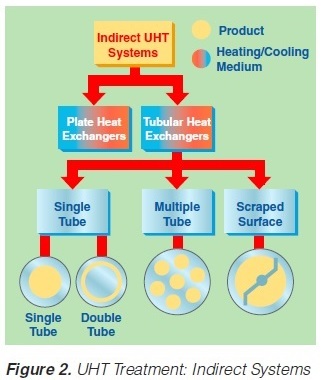
A heat exchange surface separates the product from the heating or cooling media in indirect UHT systems. The heating medium may be either steam or superheated water (Figure 2). A time-temperature diagram for indirect systems is shown in
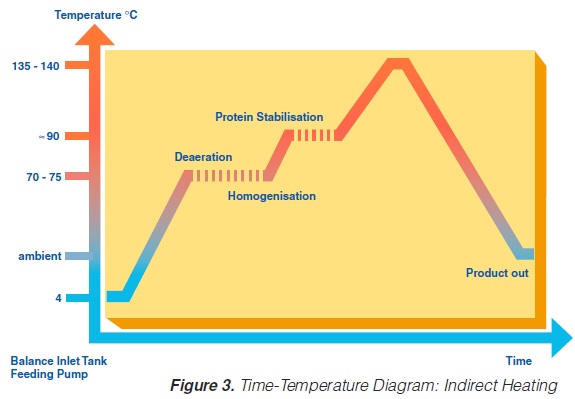
figure 3. Typically, the product enters the steriliser via a balance inlet tank and a centrifugal feeding pump at about 4°C. Subsequently, it is heated to 70 -75°C, at which temperature the product is homogenised. For milk homogenisation, pressures of 200 to 250 kg/cm2 (150 to 200 at the first stage, and about 50 kg/cm2 at the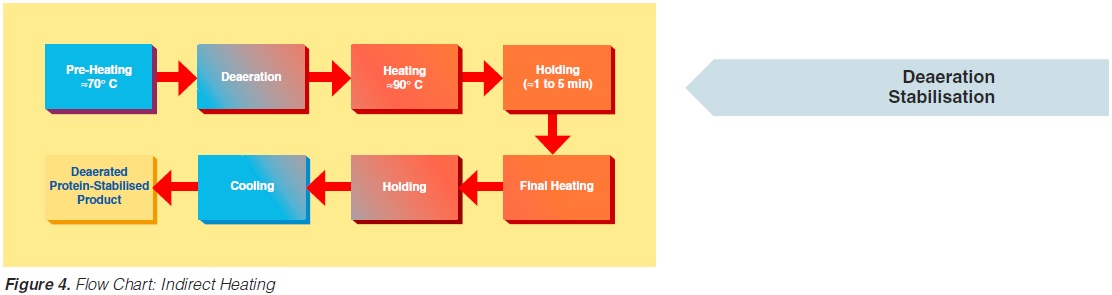 second) are often used. The omogeniser is a positive piston pump which pushes the product through the remaining equipment directly to the aseptic filling machine or into an aseptic tank for subsequent filling. Sterilisation temperatures are usually between 135°C and 140°C. Holding times between 2 and 6 seconds at the sterilisation temperature are common. Though more expensive, downstream (aseptic) homogenisation results in better flavour characteristics for some products. In order to lower the oxygen content of the product, deaeration can be introduced prior to upstream homogenisation. Milk entering the steriliser is normally saturated with oxygen. Since indirect working sterilisers are closed systems, the incoming and outgoing oxygen content of the product is the same. Depending upon temperature, milk may contain 6 to 9 ppm of oxygen (ca 7ppm), (168). Passing a vacuum chamber (deaerator) at high temperature prior to homogenisation (~ 70°C), the oxygen
second) are often used. The omogeniser is a positive piston pump which pushes the product through the remaining equipment directly to the aseptic filling machine or into an aseptic tank for subsequent filling. Sterilisation temperatures are usually between 135°C and 140°C. Holding times between 2 and 6 seconds at the sterilisation temperature are common. Though more expensive, downstream (aseptic) homogenisation results in better flavour characteristics for some products. In order to lower the oxygen content of the product, deaeration can be introduced prior to upstream homogenisation. Milk entering the steriliser is normally saturated with oxygen. Since indirect working sterilisers are closed systems, the incoming and outgoing oxygen content of the product is the same. Depending upon temperature, milk may contain 6 to 9 ppm of oxygen (ca 7ppm), (168). Passing a vacuum chamber (deaerator) at high temperature prior to homogenisation (~ 70°C), the oxygen
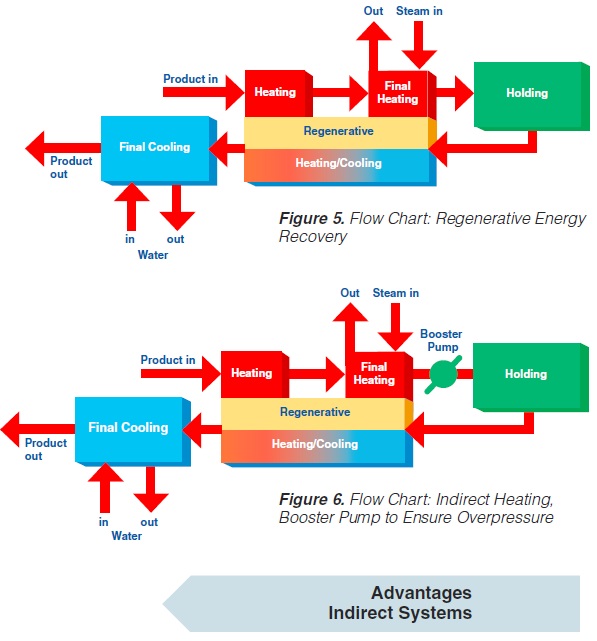
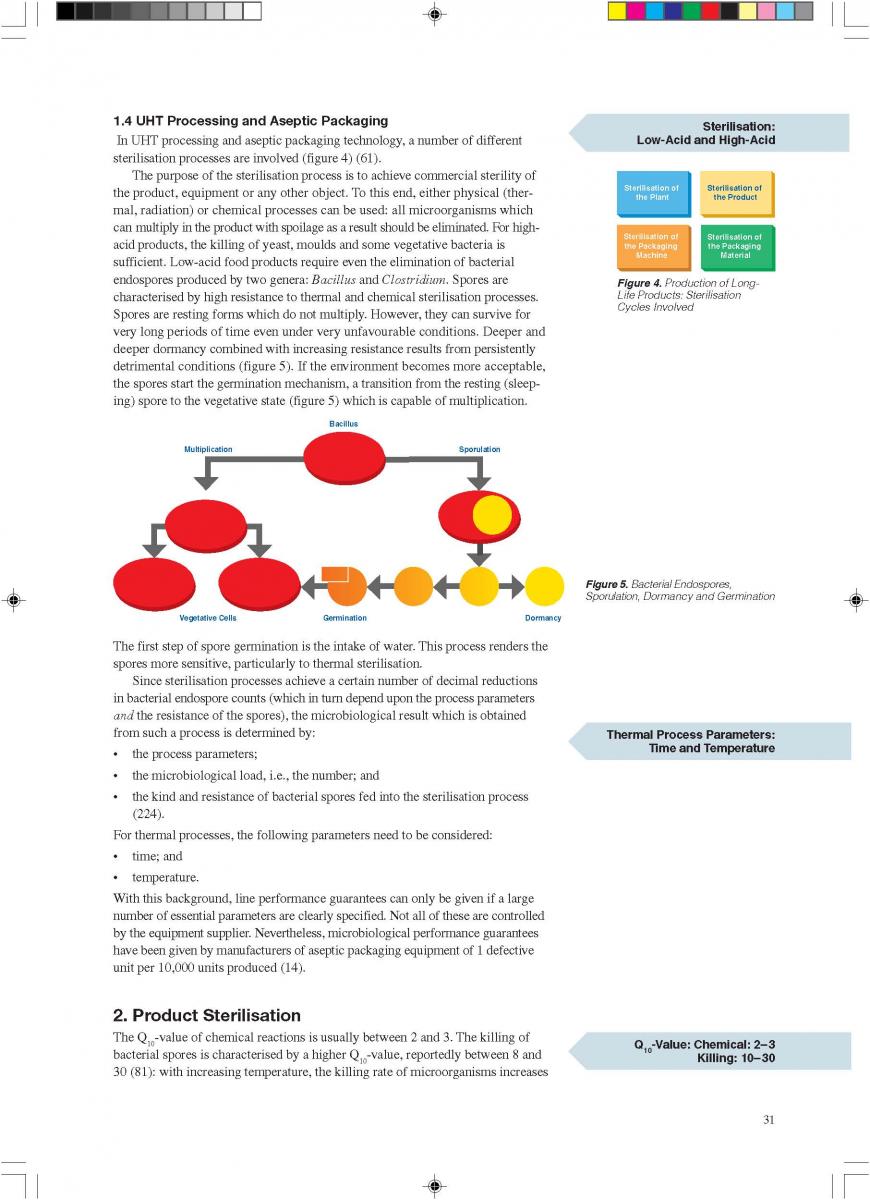
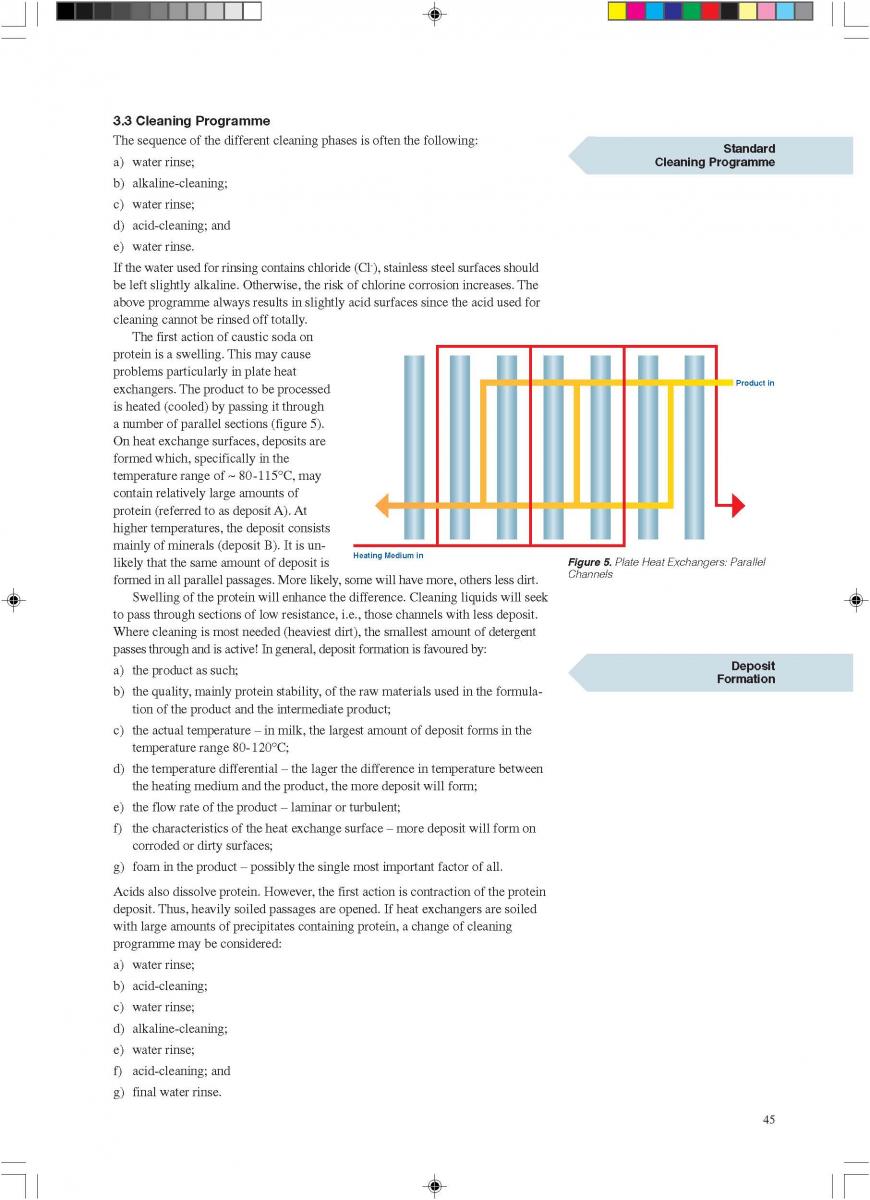
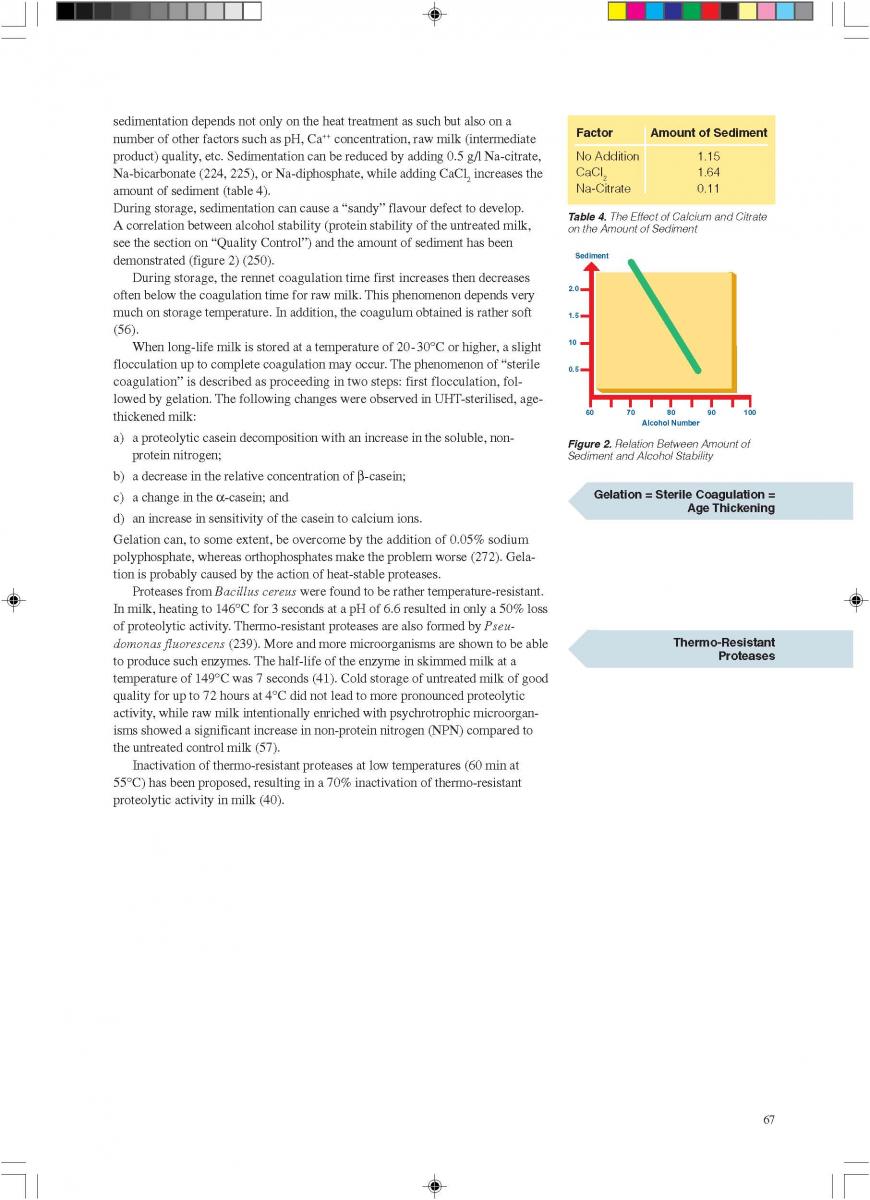
content can be reduced to about < 1 to 3 ppm (0.3 - 0.9 ppm), (168). The degree of deaeration depends upon the temperature and the underpressure applied in the process. During heat processing, deposits form on heat exchange surfaces, particularly in the temperature range > 80°C. In order to reduce this deposit formation and thus prolong the running time, a holding cell can be introduced: the product (milk) is held for a few minutes at a temperature of about 90°C (Figure 4). Indirect systems offer good possibilities for regenerative energy recovery: the incoming productis heated by the outgoing.
013.Direct UHT systems
Direct UHT Systems
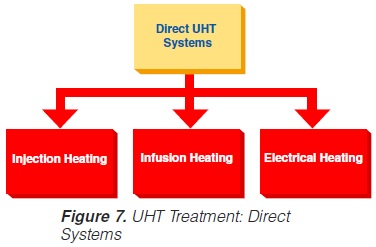 Direct UHT plants feature direct contact between the heating medium and the product. The heating medium is usually steam but electrical heating (“Elecster”, “Ohmic”) has been introduced to a very limited extent(figure 7).
Direct UHT plants feature direct contact between the heating medium and the product. The heating medium is usually steam but electrical heating (“Elecster”, “Ohmic”) has been introduced to a very limited extent(figure 7).
Electrical heating features the passage of an electrical current through the product. Although it is also used for liquids, the system is mainly intended to be applied in the sterilisation of products containing particles. Commercial application is very limited although a number of plants have been installed in the research and development departments of some major food and animal-feed processing companies. One problem is the difference in electrical resistance which can exist between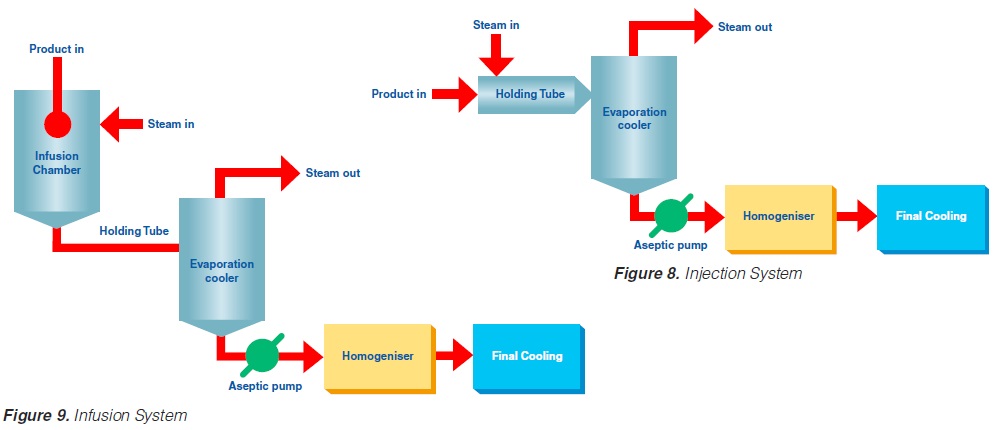 the liquid and solid phases. Other problems generally associated with the processing of products containing particles are those of separation during processing and aseptic transfer, and mechanical damage to particles softened by processing.
the liquid and solid phases. Other problems generally associated with the processing of products containing particles are those of separation during processing and aseptic transfer, and mechanical damage to particles softened by processing.
 Injection implies direct steam injection into the product. In infusion plants, the product is infused into a steam chamber.
Injection implies direct steam injection into the product. In infusion plants, the product is infused into a steam chamber.
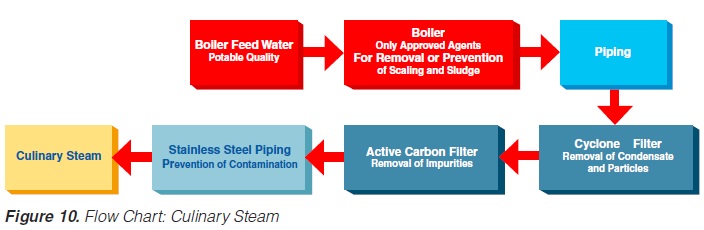
 Injection and infusion systems must be operated with culinary steam.
Injection and infusion systems must be operated with culinary steam.
The minimum requirements outlined in figure 10 must be observed for the steam in such plants (not necessary for electrical heating). Indirect working plants should also use culinary steam especially if they are sterilised with steam.
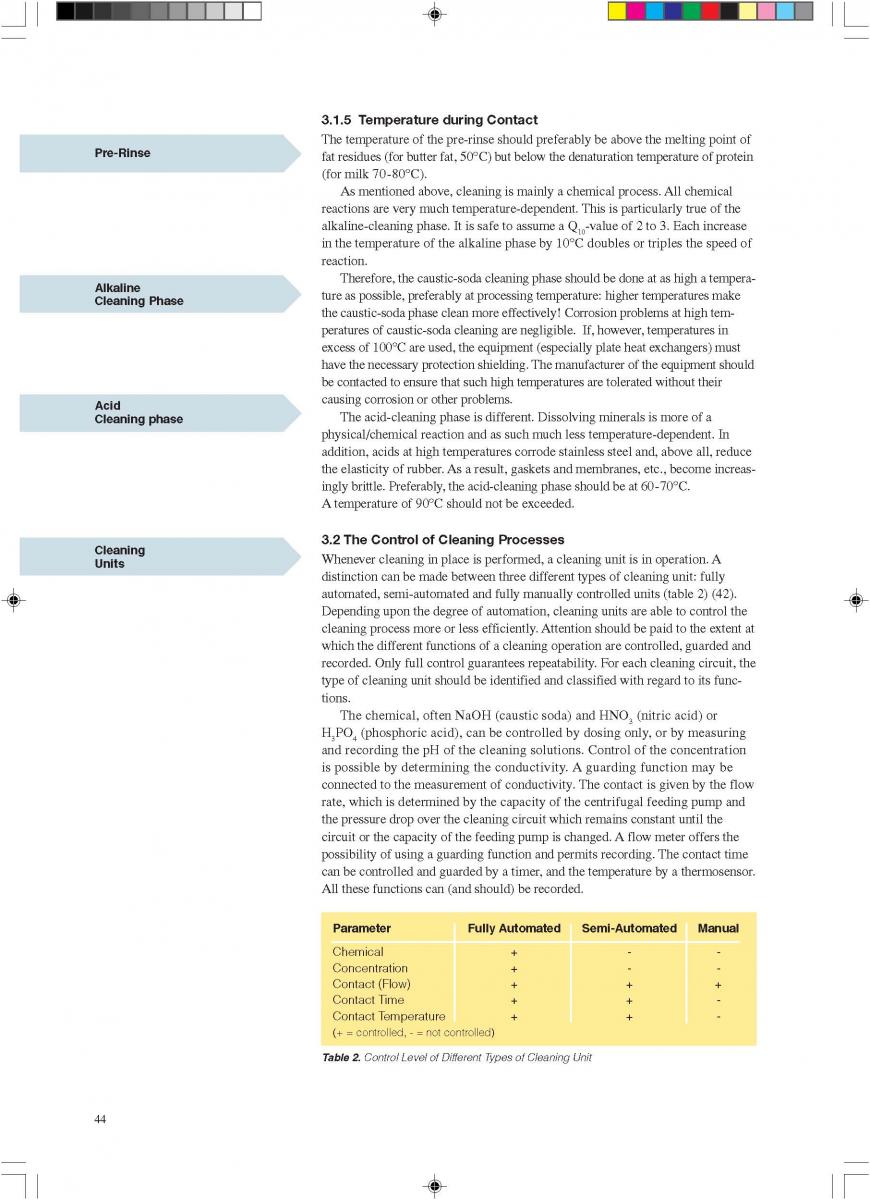
The US Department of Health and Human Services (17) lists the following requirements for culinary steam:
1. Boiler Feed Water: If boiler feed water is treated, the process must be under the supervision of trained personnel. Only compounds complying with Section 173.310 of 21 CFR (258) may be used to prevent corrosion and scale.
2. Boiler Operation: A supply of clean, dry saturated steam is necessary for proper equipment operation. It is recommended that periodic analysis be made of condensate samples.
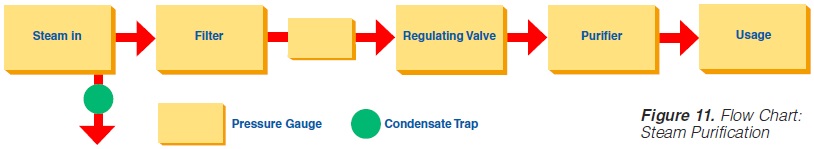
3. Piping Assemblies: The steam supply line should be as shown in figure 11.
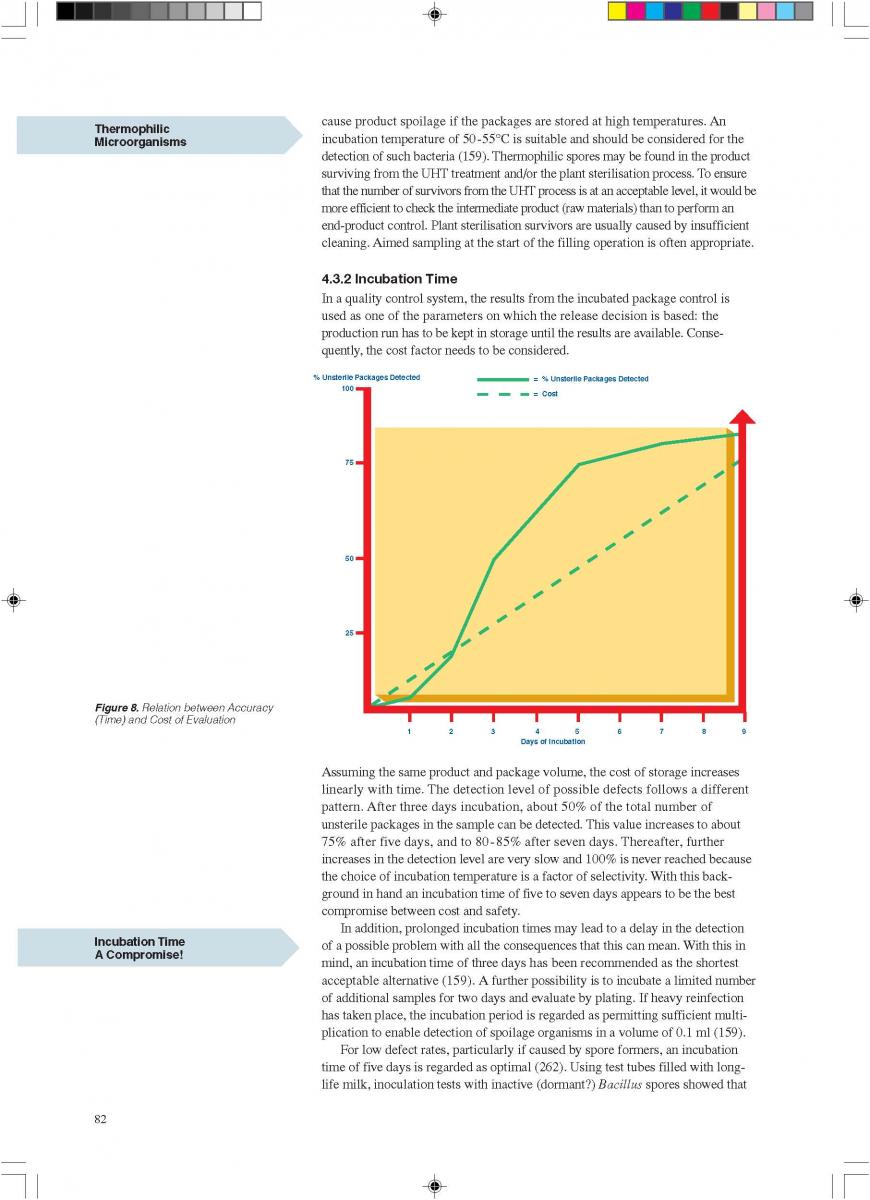
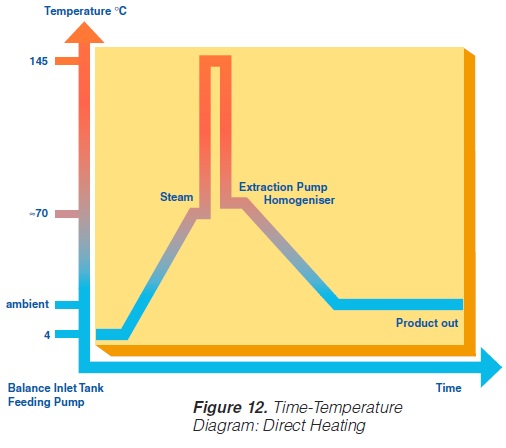 A typical time-temperature diagram for direct heating is shown in figure 12.
A typical time-temperature diagram for direct heating is shown in figure 12.
The product enters the steriliser via a balance inlet tank and a centrifugal feeding pump at a temperature of about 4°C. It is heated by plate or tubular heat exchangers to about 70°C. At this stage, steam is injected into the product or the product is infused into a steam chamber. Steam condensation increases the temperature almost instantaneously (~ 0.1 sec in injection and ~ 0.25 sec in infusion systems) to the sterilisation temperature which is typically between 145°C and 150°C. The average holding time at the sterilisation temperature is usually around 4 seconds. In both the injection and infusion processes, water condenses in the product and dilutes it. Depending on the temperature difference, an increase in volume of about 10% results. This water must be subsequently removed. The outlet of the holding cell connects to a vacuum chamber. To prevent boiling in the product holding cell, a sufficient overpressure by a suitable restriction device must be introduced. Being exposed to underpressure, the product starts boiling vigorously and steam is flushed off. Careful adjustment of the injection (infusion) temperature and the underpressure in the vacuum chamber guarantees the same dry matter content of the incoming and outgoing product. The resulting pressure drop requires the installation of an aseptic extraction pump for further product transportation. In order to avoid an accumulation of product in the expansion cooler or its emptying, the capacity of both the product feeding pump and the extraction pump at the outlet of the expansion cooler must be carefully
matched.
Cavitation forces during steam condensation and the boiling in the expansioncooler destabilise milk protein and fat. To compensate this effect requires downstream homogenisation which has to be done under aseptic conditions. Homogenisation pressures for milk are usually 200 to 250 kg/cm2 (150 to 200
kg/cm2 at the first stage and about 50 kg/cm2 at the second). The homogeniser pushes the product through the final cooling section of the steriliser, either into a sterile tank or directly to the aseptic filling machine.
In the expansion cooler, not only water is removed from the product but also all other volatile compounds. In addition, the vacuum chamber functions as a very effective deaerator that removes oxygen and other dissolved gases, mainly carbon dioxide (CO2). As a consequence, the freezing point increases. At the outlet of the expansion cooler, the oxygen content of milk is down to ~ 0.1 ppm.
 Advantages of injection and infusion heating are:
Advantages of injection and infusion heating are:
a) a lower total heat load, as a result of which fewer chemical changes are inflicted on the product;
b) less scaling, particularly in the temperature range of 70°C and above resulting in longer production runs (less frequent cleaning and sterilisation);
c) the low oxygen content in the product increases the stability of some vitamins and, during storage, reduces flavour changes caused by oxidation;
d) more suitable for viscous products.
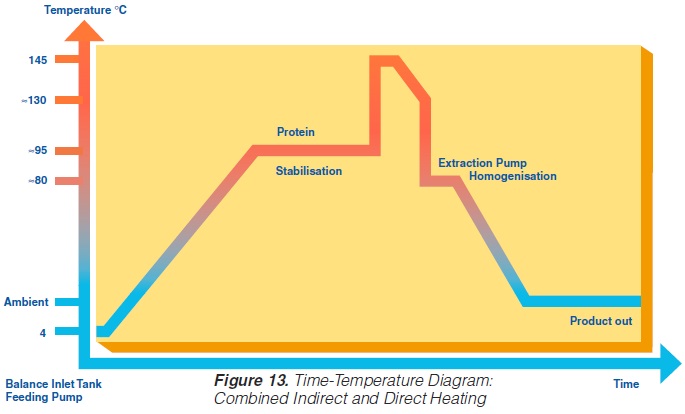
Recently, special UHT heat exchangers have been developed combining direct and indirect heating. In one assembly, tubular heating and steam injection have been combined. The product enters at ~4°C and is heated to 95°C by tubular heat exchangers. It then passes a holding cell at the same temperature to stabilise the protein. Steam injection raises the temperature almost instantly to 140-150°C. The product is held at this temperature for a few seconds before being cooled down. Pre-cooling is performed in a tubular heat exchanger where the heat energy is utilised for regenerative heating. The injected steam is flushed off as vapour in a vacuum cooler. The temperature drops to 80°C. Aseptic homogenisation is needed. Subsequently, the product is cooled down to ambient and filled aseptically.
014. Plant Sterilisation
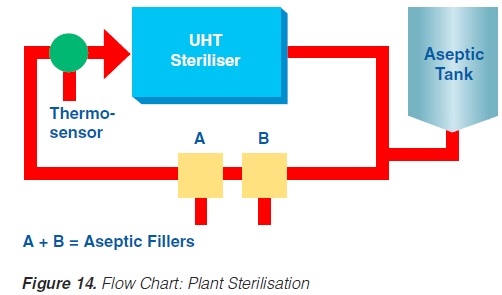 Prior to production, the equipment must be sterilised. This can be achieved either by superheated water or by steam. Because of the expansion and infusion vessels, direct working systems must be sterilised by steam. It is essential that the temperature controller/guarding sensor is placed at the most sensitive spot, usually somewhere in the return.
Prior to production, the equipment must be sterilised. This can be achieved either by superheated water or by steam. Because of the expansion and infusion vessels, direct working systems must be sterilised by steam. It is essential that the temperature controller/guarding sensor is placed at the most sensitive spot, usually somewhere in the return.
If superheated water is used to sterilise the plant, the temperature, time, and flow rates are critical (142). Possible air pockets and the interface between the product steriliser and the aseptic tank circuit are significant. Air is effectively removed from the system if the flow rate is > 1.5 m/second.
An additional sterilisation circuit is required in installations operating through an aseptic tank. The sterilisation medium is always steam. In steam sterilisation processes, a possible problem is condensate which must be removed from the system.
Sterilisation of the aseptic filling equipment is always performed separately either by heat alone or by chemicals and heat. Attention should be paid to the interface between the product line and the filler.
02.Aseptic packaging
Requires 90 POINTS in the General category.021. General
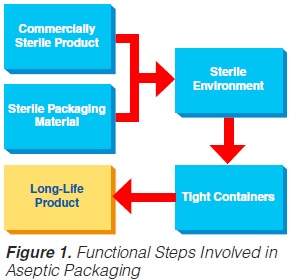 UHT processing results in a commercially sterile product. The task of the aseptic packaging operation is to:
UHT processing results in a commercially sterile product. The task of the aseptic packaging operation is to:
a) maintain the high microbiological quality of the product for the length of its intended shelf life; and
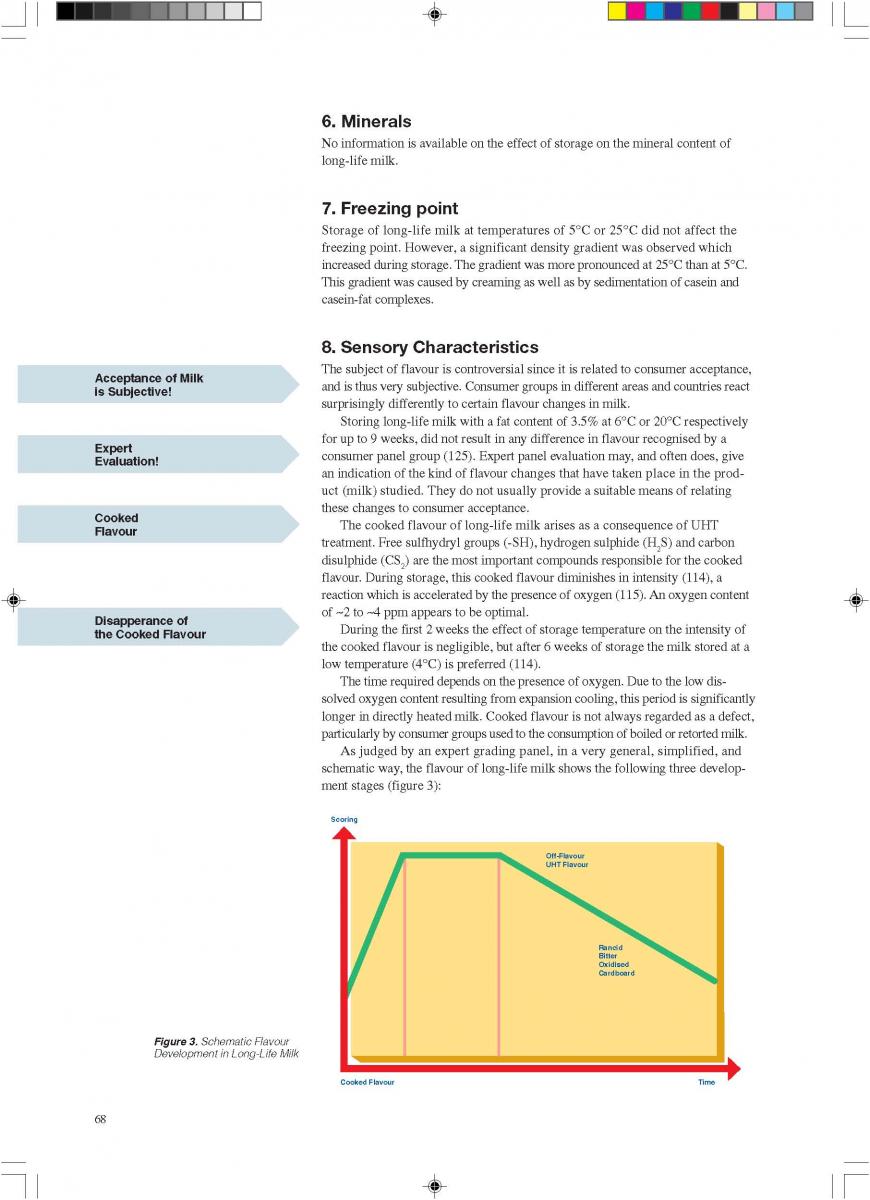
b) retain consumer acceptance with regard to the flavour, texture and nutritional value of the product during its promised shelf life.
Four different principles of aseptic packaging are distinguished (74):
a) filling in a sterile working area;
b) aseptic filling into pre-formed containers;
c) aseptic filling into pre-formed sterile containers; and
d) the aseptic form-fill-and-seal method.
By and large, only the aseptic form-fill-and-seal method will be covered, which is also the best and most successful technology. Such an aseptic packaging operation requires several functional steps (figure 1) (60, 61, 74, 86).
022. Sterilisation of the Packaging Material
2.Sterilisation of the Packaging Material
In aseptic packaging procedures, sterilisation of the packaging material (food contact surface) is achieved with few exceptions by chemical means (61). By far the most common chemical used for this purpose is hydrogen peroxide (H2O2) (93, 142). Of importance are the microbiological efficiency of the sterilisation process and the elimination of the chemical which might get into the filled product as a residue.
Depending on the make of the aseptic packaging equipment, different means of applying the sterilant are used: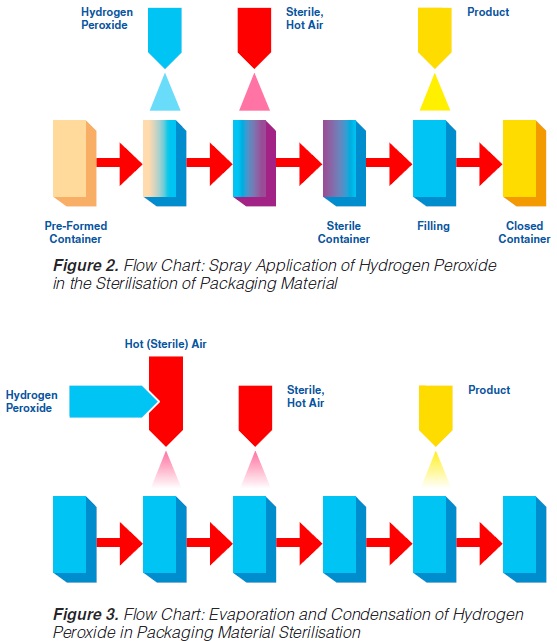
1) spray;
2) vapour;
3) roller systems;
4) immersion bath, etc.
2.1 Spray Application
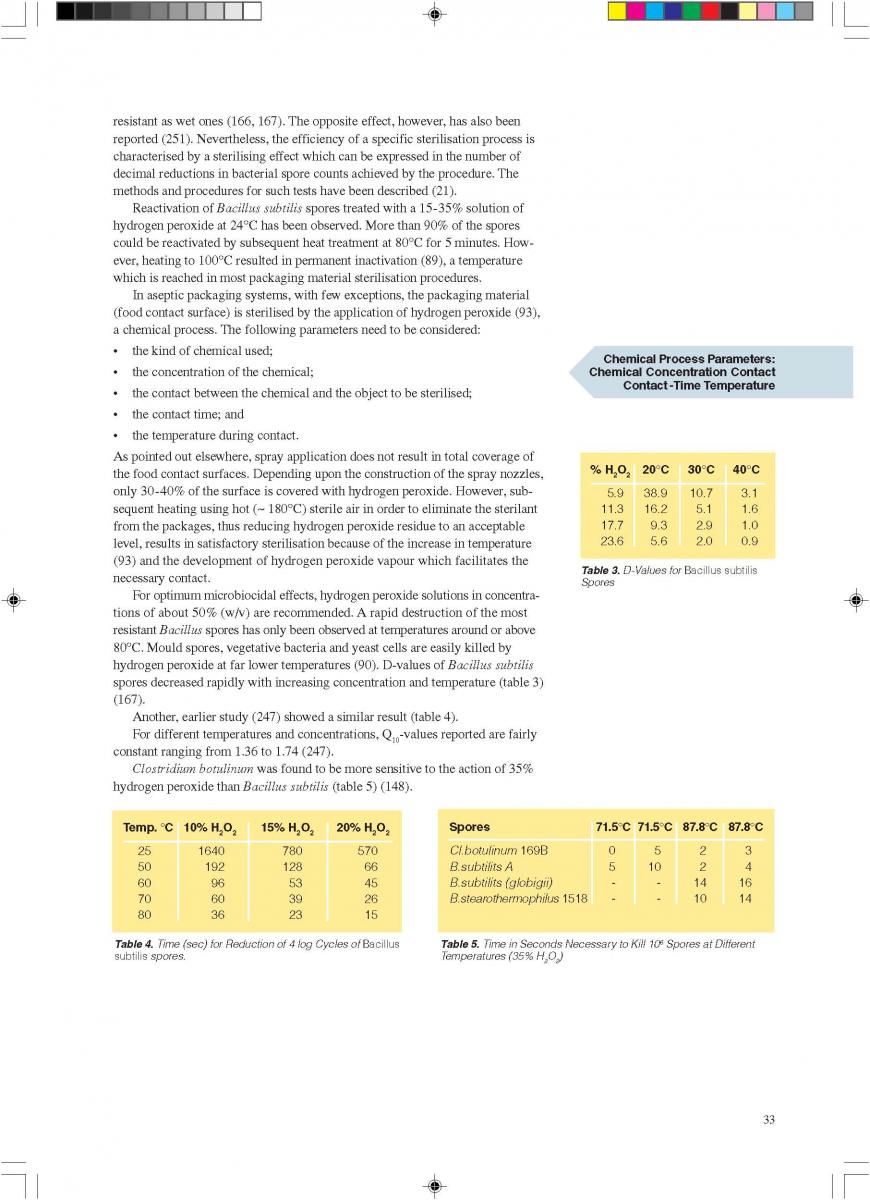
The spraying (“fogging”) of hydrogen peroxide is used in some aseptic packaging systems which operate intermittently and use pre-formed blanks (61, 74, 93, 171).
A certain amount of H2O2 is sprayed into each pre-erected container through a spray nozzle (figure 2). For proper function (sterilisation), the food contact surface of the container needs to be covered completely with the spray solution. Because of the hydrophobic characteristics of plastic materials in general and polyethylene in particular, it has been found (91, 145) that only 20-30% of the carton surfaces are wetted. In spite of this, good killing effects were achieved: up to 6 log reductions when tested with Bacillus subtilis spores have been reported (145), probably because of the subsequent evaporation.
Hot sterile air is blown into the container to attain the temperature necessary for the sterilisation process and to remove the H2O2 from the food contact surface. Using sterile air at
180°C for drying, 5 to 7 decimal reductions of Bacillus subtilis spores were attained (91, 113).
The killing efficiency of low concentrations (~ 0.5% or less) of hydrogen peroxide was greatly enhanced by the simultaneous use of ultraviolet radiation (44, 45).
2.2 Application by Vapour
In systems applying hydrogen peroxide by vaporisation, liquid H2O2 is injected into a stream of hot (sterile) air, then vaporised and condensed on the surfaces to be sterilised (93). A better coverage
of surfaces is obtained since the condensing droplets are smaller. Subsequently, the hydrogen peroxide is heated and evaporated by blowing hot, sterile air into the containers (figure 3).
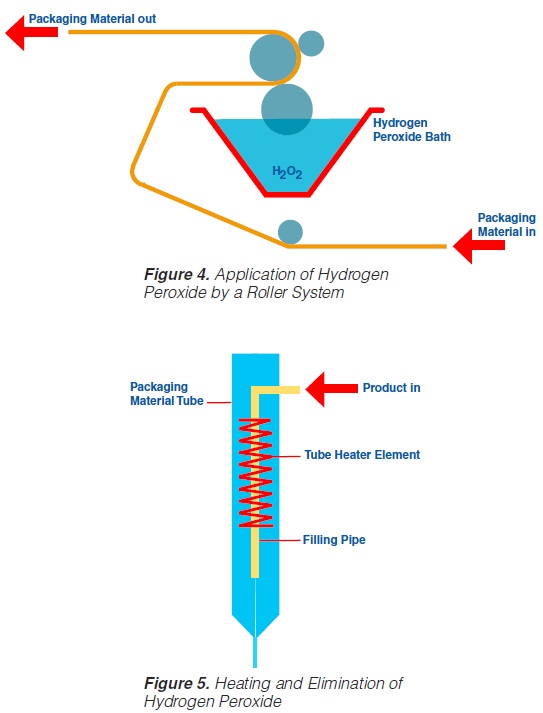
2.3 Application by Roller System
Roller systems (figure 4) permit the application of liquid hydrogen peroxide on to flat food contact surfaces (59, 60, 61, 74, 93). Packaging material sterilisation thus becomes possible before the containers are formed (figure 4).
In order to obtain a film of the water like H2O2 liquid covering the total food contact surface, a wetting agent – about 0.2 to 0.3% of PSM, (polyoxyethylenesorbitan-
monolaurate), or equivalent is recommended - has to be added to the hydrogen peroxide. After application of the sterilant on to the food contact surface, the packaging material web is formed into a tube and sealed longitudinally. The actual sterilisation requires the hydrogen peroxide covering the
packaging material food contact surface to be at a high temperature. An electrical element (“tube heater”) provides the temperature necessary (105-110°C) for the sterilisation process and, simultaneously, eliminates the H2O2 (figure 5).
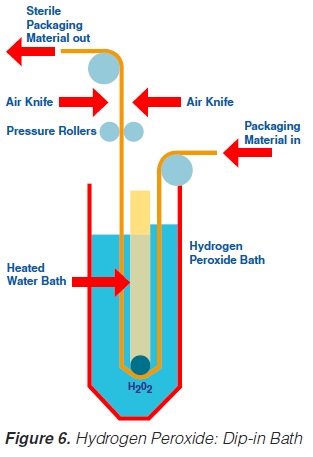
2.4 Use of an Immersion Bath
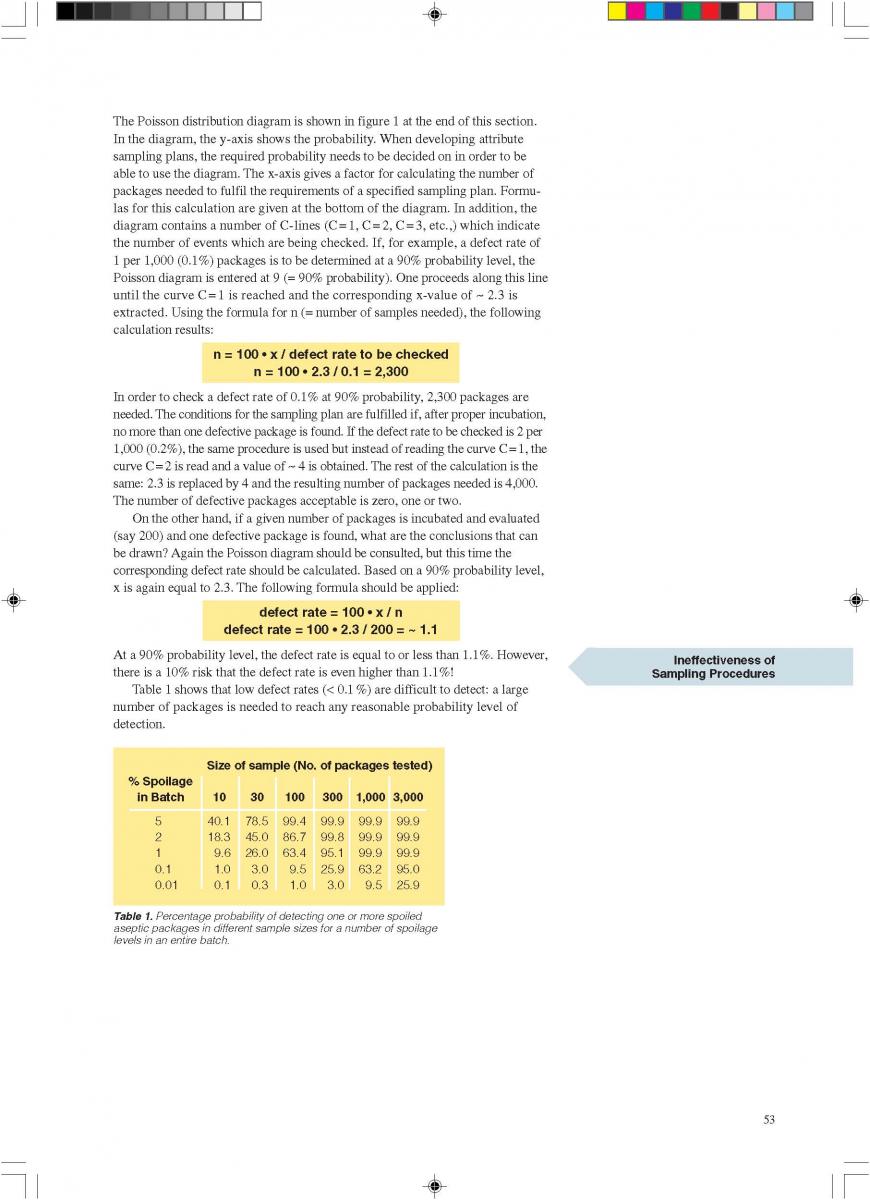
Likewise, the use of a hydrogen peroxide bath permits sterilisation of a plane packaging material web (59, 61, 69, 73, 74, 93, 171) prior to the actual forming of the container. The packaging material is sterilised by its passage through liquid hydrogen peroxide. The temperature necessary for the sterilisation process is obtained by heating the hydrogen peroxide solution indirectly by means of a water bath which is placed inside the H2O2 bath (figure 6). A concentration of 30%, a temperature of 70°C and an exposure time of about 10 seconds are necessary to achieve sufficient killing of bacterial endospores (74). In some models, the hydrogen peroxide is removed from the packaging material:
a) by a pair of pressure rollers that squeeze the excess chemical back into the hydrogen peroxide bath; and
b) by a pair of air knives that blow hot, sterile air on to both sides of the packaging material web.
After passage through the bath and removal of the hydrogen peroxide, the sterile, flat packaging material web is formed into a tube and sealed longitudinally.
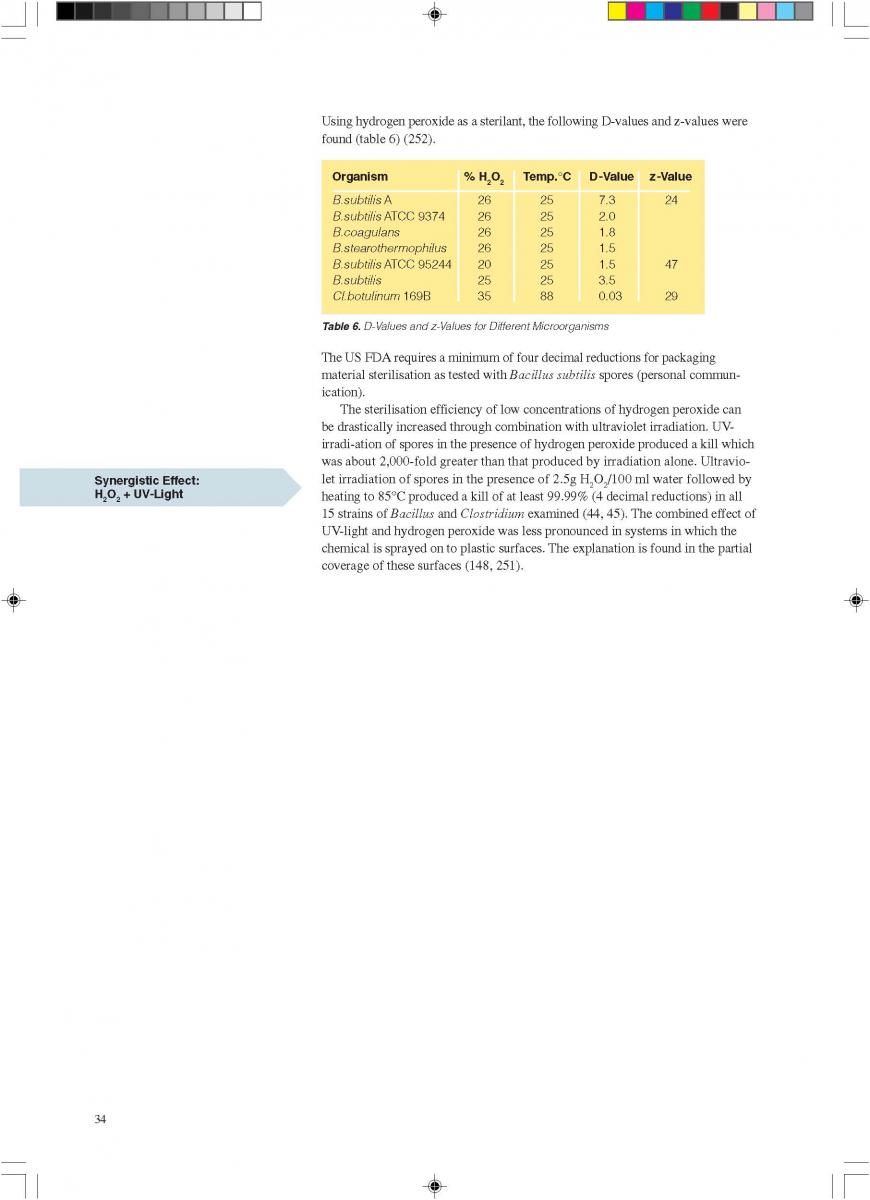
04. H2O2 as a sterilant



05. Packaging material


06. Application of microbiology to UHT processing and aseptic packaging

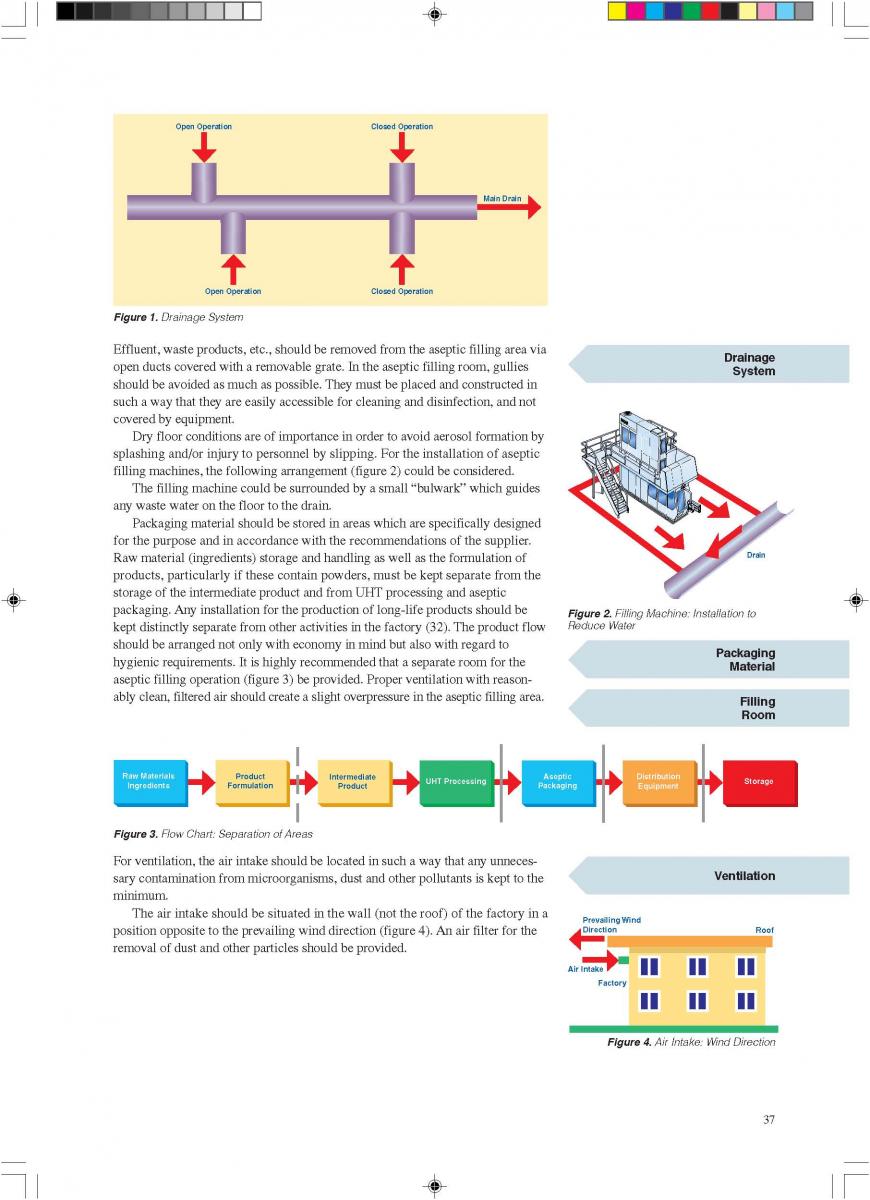
07. Installation


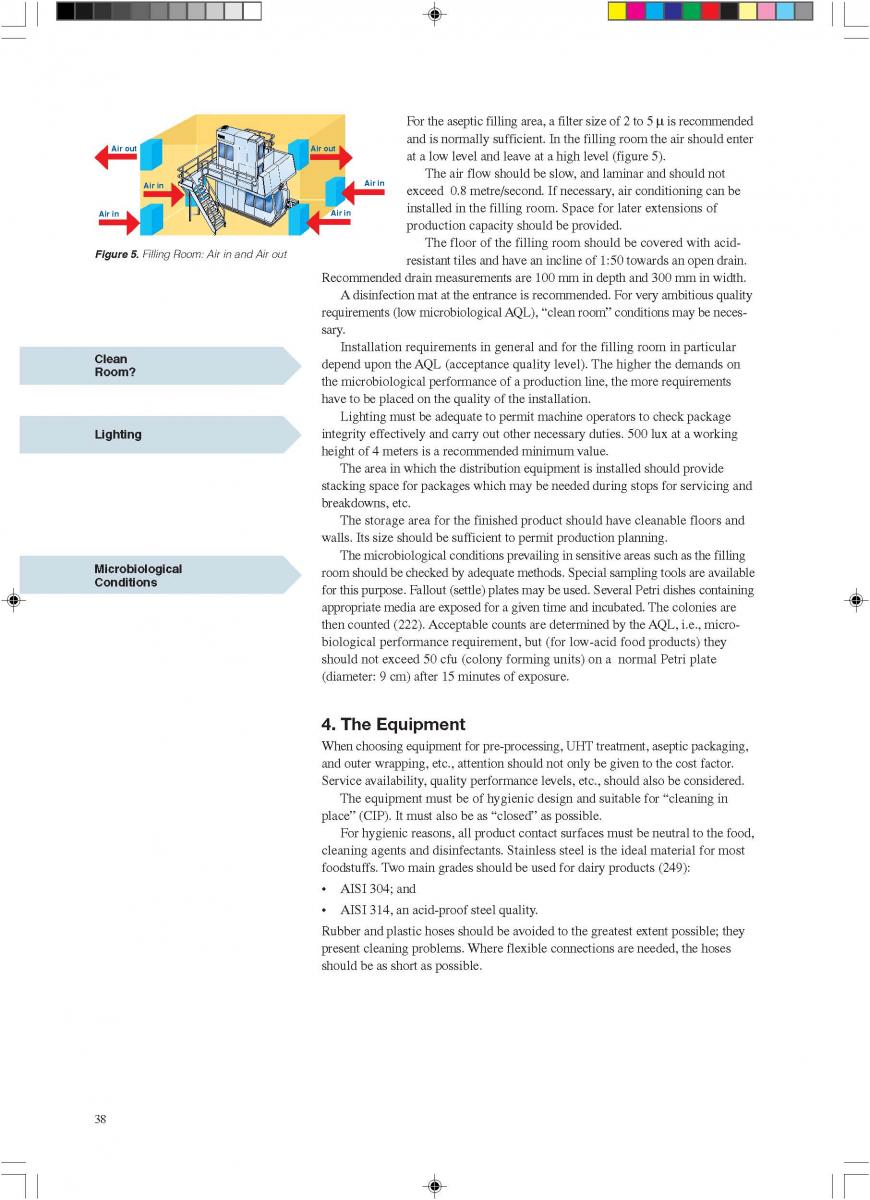
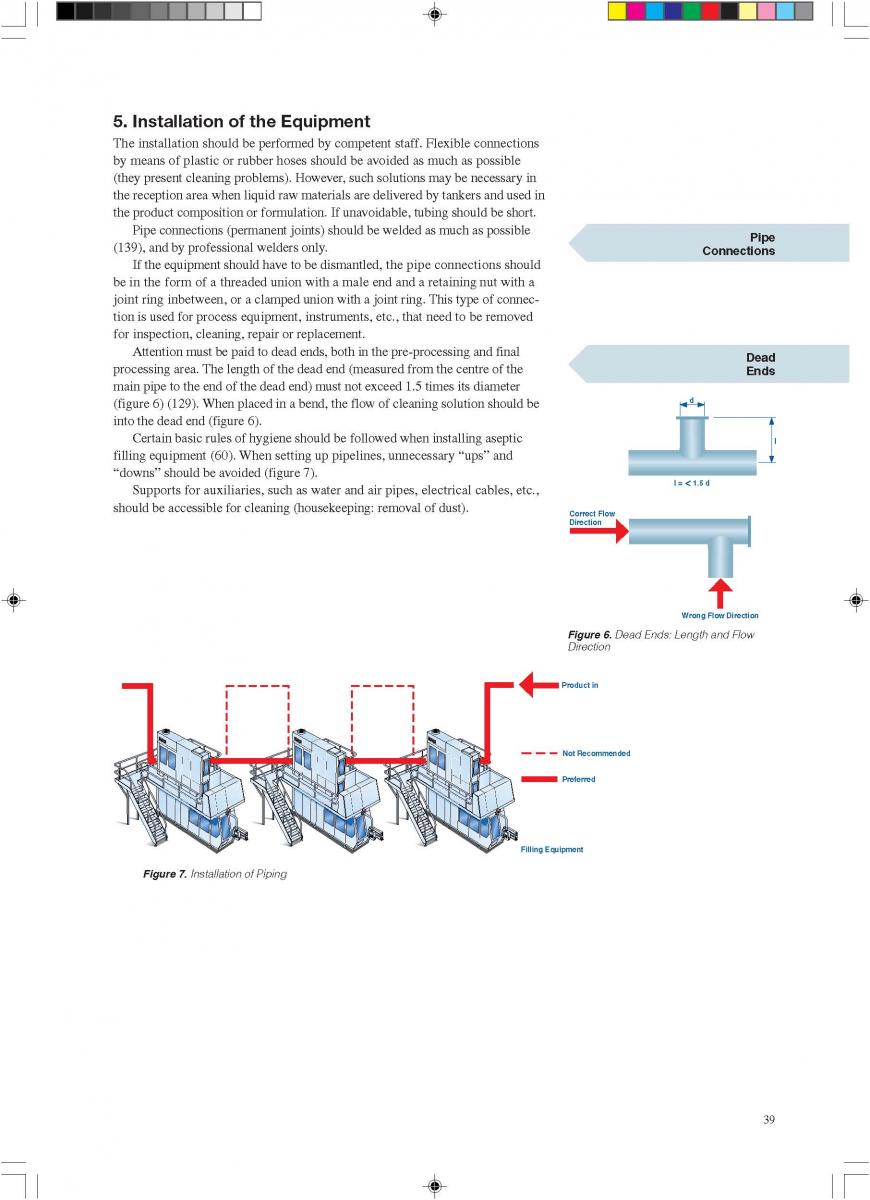
08. Cleaning and house keeping






09. Commissioning
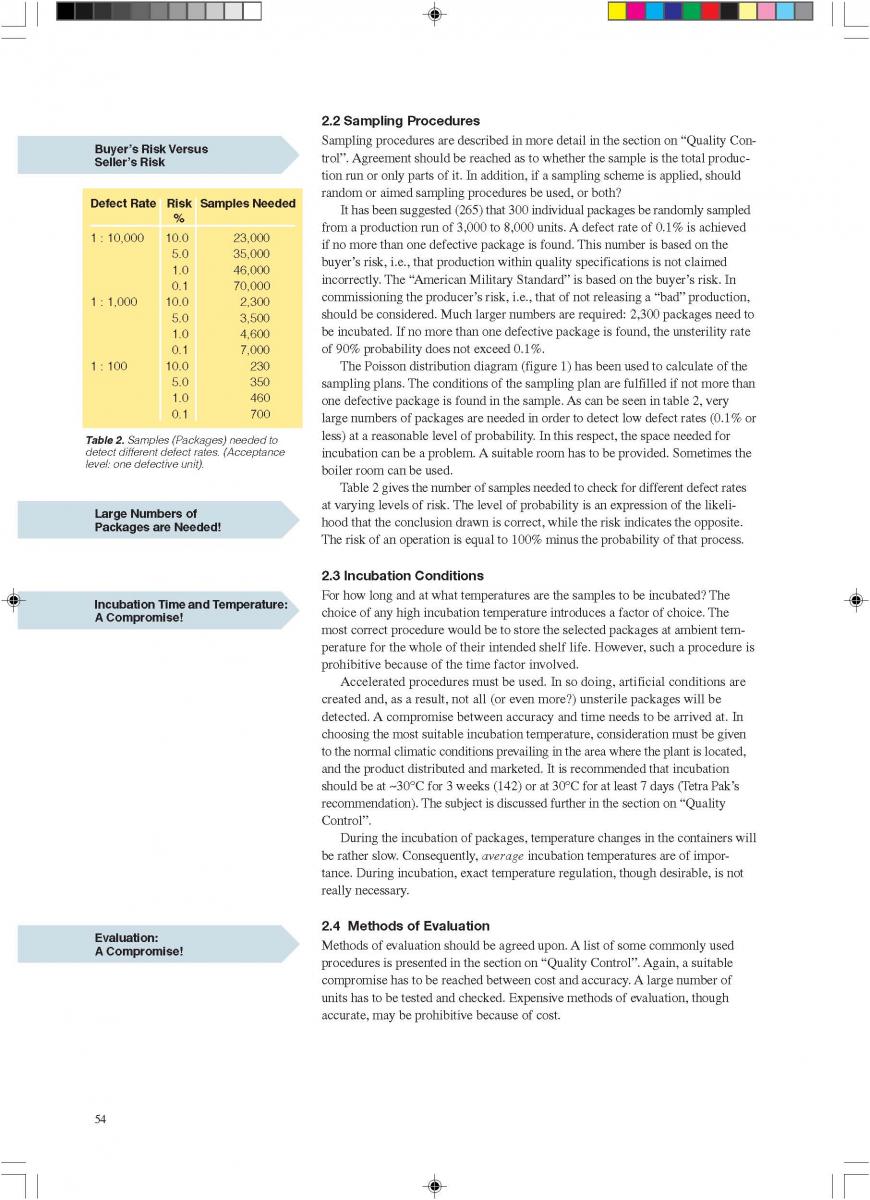
10. Changes during milk processing


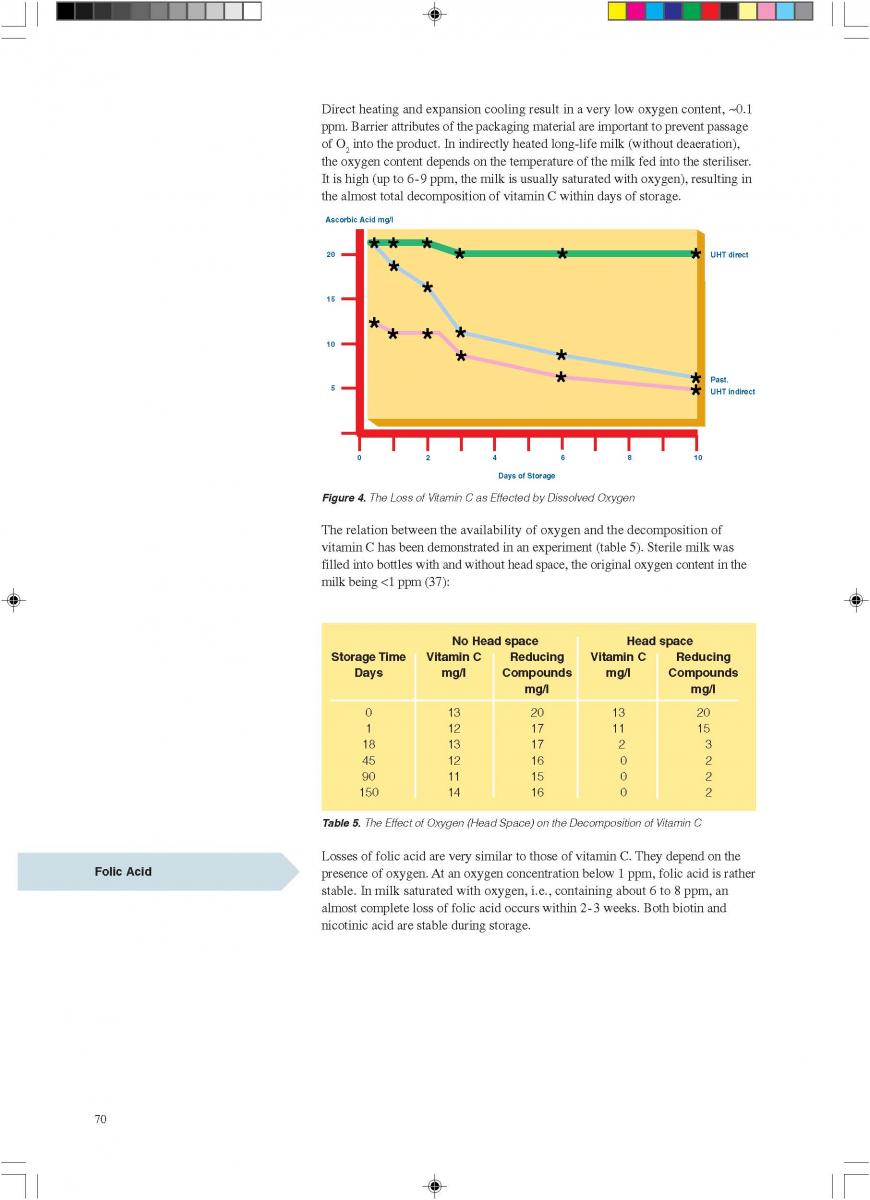
11. Product changes during storage



12. Quality control of aseptic product-Part1


12. Quality control of aseptic product-Part2





Bacteria, virus an human life
 Do you know what live in a tiny water? How many bateria and viruses are affeting human's lives. Now we granted soap and bathe actually took a long time to be accepted in human lives. Our skin and hair will appear as the valleys and huge caves to the microbes. Bacteria can cause illness and allergy, they can also eat the flesh of human being. Some bacteria can even explore in the brain and break the brain. And the -20 C refrigerator, which we think being safe, actually is some bacteria's living bed and they can kill us if we eat the stored foods without heating. Some bacteria still live in the UHT product quietly without being feeling or seen. Some can do benefit to us such as making cheese and yoghurt. The toxin of clostridium botulilum is 1000 times as the most dangerous snake in the world, but they can remove the skin and face's wrinkles. Some popular microbes such as those who can make people catch cold, kill people in a 90% probability which is Ebola virus, phages which can make fermenting failing and those which can make tulips more beatiful ...
Do you know what live in a tiny water? How many bateria and viruses are affeting human's lives. Now we granted soap and bathe actually took a long time to be accepted in human lives. Our skin and hair will appear as the valleys and huge caves to the microbes. Bacteria can cause illness and allergy, they can also eat the flesh of human being. Some bacteria can even explore in the brain and break the brain. And the -20 C refrigerator, which we think being safe, actually is some bacteria's living bed and they can kill us if we eat the stored foods without heating. Some bacteria still live in the UHT product quietly without being feeling or seen. Some can do benefit to us such as making cheese and yoghurt. The toxin of clostridium botulilum is 1000 times as the most dangerous snake in the world, but they can remove the skin and face's wrinkles. Some popular microbes such as those who can make people catch cold, kill people in a 90% probability which is Ebola virus, phages which can make fermenting failing and those which can make tulips more beatiful ...
Movie of bacteria and virus with people
 Do you know what live in a tiny water? How many bateria and viruses are affeting human's lives. Now we granted soap and bathe actually took a long time to be accepted in human lives. Our skin and hair will appear as the valleys and huge caves to the microbes. Bacteria can cause illness and allergy, they can also eat the flesh of human being. Some bacteria can even explore in the brain and break the brain. And the -20 C refrigerator, which we think being safe, actually is some bacteria's living bed and they can kill us if we eat the stored foods without heating. Some bacteria still live in the UHT product quietly without being feeling or seen. Some can do benefit to us such as making cheese and yoghurt. The toxin of clostridium botulilum is 1000 times as the most dangerous snake in the world, but they can remove the skin and face's wrinkles. Some popular microbes such as those who can make people catch cold, kill people in a 90% probability which is Ebola virus, phages which can make fermenting failing and those which can make tulips more beatiful ...
Do you know what live in a tiny water? How many bateria and viruses are affeting human's lives. Now we granted soap and bathe actually took a long time to be accepted in human lives. Our skin and hair will appear as the valleys and huge caves to the microbes. Bacteria can cause illness and allergy, they can also eat the flesh of human being. Some bacteria can even explore in the brain and break the brain. And the -20 C refrigerator, which we think being safe, actually is some bacteria's living bed and they can kill us if we eat the stored foods without heating. Some bacteria still live in the UHT product quietly without being feeling or seen. Some can do benefit to us such as making cheese and yoghurt. The toxin of clostridium botulilum is 1000 times as the most dangerous snake in the world, but they can remove the skin and face's wrinkles. Some popular microbes such as those who can make people catch cold, kill people in a 90% probability which is Ebola virus, phages which can make fermenting failing and those which can make tulips more beatiful ...
Virus relevant Movie:I am legend
I Am Legend is a 2007 American post-apocalyptic science fiction horror film directed by Francis Lawrence and starring Will Smith. It is the third feature film adaptation of Richard Matheson's 1954 novel of the same name, following 1964's The Last Man on Earth and 1971's The Omega Man.[2] Smith plays virologist Robert Neville, who is immune to a man-made virus originally created to cure cancer. He works to create a remedy while defending himself against mutants created by the virus.
Food Safety and Microbial Quality
As one important component of quality system in food and beverage industry, HACCP plays a very effective role in assuring food safety with the precondition that people can use it properly in which practice experience and structured food safety knowledge are the cores to arm people of production unit besides the principle. Policy, guideline and supervison from the government are the other side to assure the food safety in which food safety knowledge, supervising system, and effective legal system are the huge challenges.
Bacteria which cannot be killed by UHT-Nightmare of UHT milk
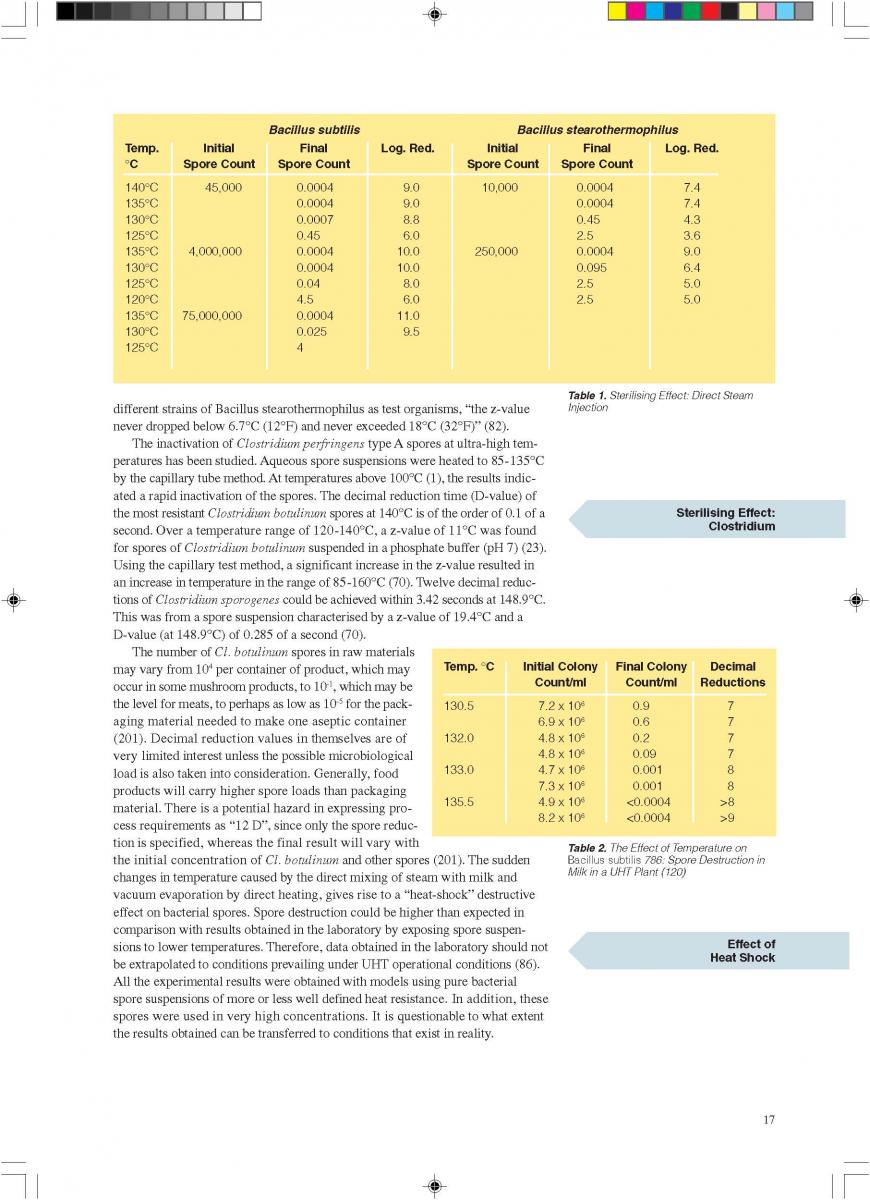
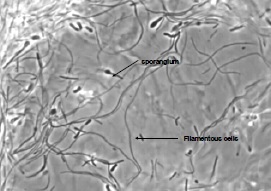 Bacillus sporothermodurans cannot be killed by UHT which contributes the whole batch products' spoilages or unsterilities. Bacillus sporothermodurans producing highly heat-resistant spores (HRS) which may survive ultra-high temperature (UHT) treatment or industrial sterilization. Molecular typing showed a heterogeneous group of farm isolates (non-HRS strains), but a clonal group of UHT isolates from diverse European countries and other continents (HRS-clone) suggesting a common source. During a survey of Belgian dairy farms for the presence of potentially highly heat-resistant spore formers, high numbers of these spores were detected in filter cloth, green crop and fodder samples. The strain collection showed a high taxonomic diversity with 18 potentially new species and with Bacillus licheniformis and Geobacillus pallidus as predominating species overall. Seventeen B. sporothermodurans isolates were identified, mainly originating from feed concentrate. Heat resistance studies showed the UHT resistance of B. sporothermodurans spores present in industrially contaminated UHT milk, but a lower heat resistance of laboratory-grown strains (HRS and non-HRS). Hydrogen peroxide, used as sanitizer in the dairy industry, was found to induce higher heat resistance of laboratory-grown B. sporothermodurans strains to a certain level. This indicates that sublethal stress conditions may affect the heat resistance. By transmission electron microscopy, structural differences at the spore level were found between HRS and non-HRS strains. The data indicate that the attainment of extreme heat resistance is rather multifactorial.
Bacillus sporothermodurans cannot be killed by UHT which contributes the whole batch products' spoilages or unsterilities. Bacillus sporothermodurans producing highly heat-resistant spores (HRS) which may survive ultra-high temperature (UHT) treatment or industrial sterilization. Molecular typing showed a heterogeneous group of farm isolates (non-HRS strains), but a clonal group of UHT isolates from diverse European countries and other continents (HRS-clone) suggesting a common source. During a survey of Belgian dairy farms for the presence of potentially highly heat-resistant spore formers, high numbers of these spores were detected in filter cloth, green crop and fodder samples. The strain collection showed a high taxonomic diversity with 18 potentially new species and with Bacillus licheniformis and Geobacillus pallidus as predominating species overall. Seventeen B. sporothermodurans isolates were identified, mainly originating from feed concentrate. Heat resistance studies showed the UHT resistance of B. sporothermodurans spores present in industrially contaminated UHT milk, but a lower heat resistance of laboratory-grown strains (HRS and non-HRS). Hydrogen peroxide, used as sanitizer in the dairy industry, was found to induce higher heat resistance of laboratory-grown B. sporothermodurans strains to a certain level. This indicates that sublethal stress conditions may affect the heat resistance. By transmission electron microscopy, structural differences at the spore level were found between HRS and non-HRS strains. The data indicate that the attainment of extreme heat resistance is rather multifactorial.
Biofilm changes the characteristic of microbes compromising food safety
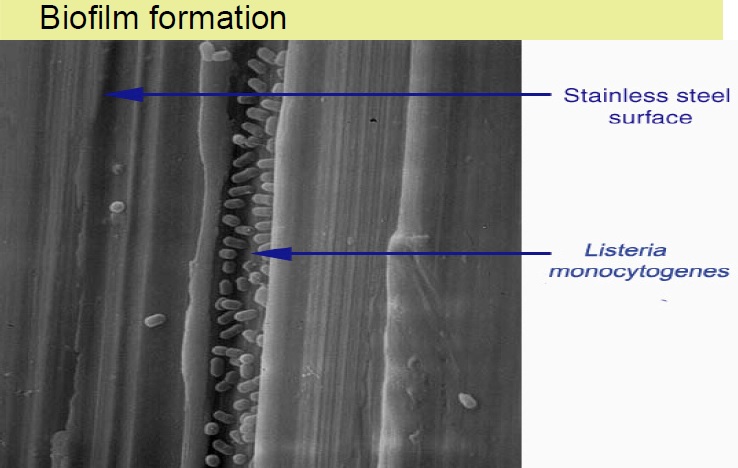 Adhesion of microorganisms to food processing equipment surfaces and the problems it causes are a matter of concern to the food industry. Biofilms have the potential to act as a chronic source of microbial contamination which may compromise food quality and represent a significant health hazard. To control these problems, it has been recognized that a greater understanding of the interaction between microorganisms and food-processing surfaces is required.Several groups have reported the ability of bacteria to attach to surfaces commonly found in the food processing environment, such as rubber and stainless steel . The increased resistance of these sessile organisms towards disinfectants, heat and sanitizing agents often exacerbates the problems caused by microbial fouling and can contribute to the inefficacy of cleaning in place systems. Development of adsorbed layers, often termed “conditioning” of a surface, is considered to be the first stage in biofilm formation and has been widely demonstrated. Because this conditioning film is likely to change the physicochemical properties of the substratum and thus to influence bacterial attachment, an understanding of these initial interactions is crucial in identifying control measures. A variety of proteins, including milk proteins, have been shown to affect bacterial adhesion to surfaces such as polystyrene; hydroxyapatite; glass, rubber, and stainless steel; silica; and medical implants. The nature of the effect appears to vary with the organism, substratum, and protein under investigation.
Adhesion of microorganisms to food processing equipment surfaces and the problems it causes are a matter of concern to the food industry. Biofilms have the potential to act as a chronic source of microbial contamination which may compromise food quality and represent a significant health hazard. To control these problems, it has been recognized that a greater understanding of the interaction between microorganisms and food-processing surfaces is required.Several groups have reported the ability of bacteria to attach to surfaces commonly found in the food processing environment, such as rubber and stainless steel . The increased resistance of these sessile organisms towards disinfectants, heat and sanitizing agents often exacerbates the problems caused by microbial fouling and can contribute to the inefficacy of cleaning in place systems. Development of adsorbed layers, often termed “conditioning” of a surface, is considered to be the first stage in biofilm formation and has been widely demonstrated. Because this conditioning film is likely to change the physicochemical properties of the substratum and thus to influence bacterial attachment, an understanding of these initial interactions is crucial in identifying control measures. A variety of proteins, including milk proteins, have been shown to affect bacterial adhesion to surfaces such as polystyrene; hydroxyapatite; glass, rubber, and stainless steel; silica; and medical implants. The nature of the effect appears to vary with the organism, substratum, and protein under investigation.Clean to remove microbes in the pipelines of dairy plant
How to clean round the bend removing completely any dairy residues in the milk or beverage transfer pipes which may be the growth bed or protection of microbes.
Guidelines for the microbiological quality of some ready-to-eat foods sampled at the point of sale
Requires 30 POINTS in the General category.Infection source of bacteria frequently found in Dairy industry
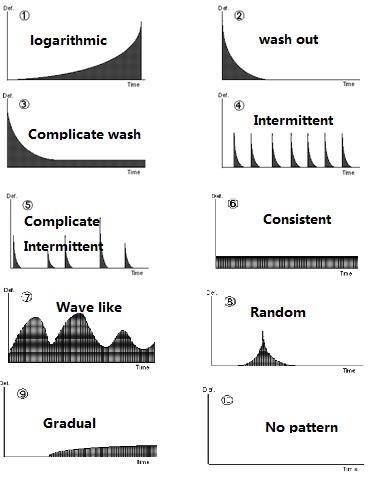 Where do the bacteria come? Using infection pattern, the question can be answered.
Where do the bacteria come? Using infection pattern, the question can be answered.
Pattern 1: logarithmic
Either the rate of infection at the source increases with time and/or substantial microbial growth takes place somewhere in the system.
One area that can be excluded/eliminated from such a scenario is faulty CIP or pre-sterilisation.
But leakage of paper tube, leakage of Valve 78, leakage of Aseptic tank, holding section of UHT and contaminated floater etc. have high probabilities.
Pattern 2: wash out
CIP/Sterilization failure linked bacteria residues often contribute such scenarios. Filling tube of Tetra Fino often give mixed cocci contamination if the tube is scratched by wrong cleaning brush, the seat of Valve A sometimes give endospores wash out if the manual clean is not carried out in a very long time. Because Escherichia can grow vigorously in organic residue, the poor CIP may result pure Escherichia flora.
Pattern 3:Complicate Wash out
Besides the cause found in wash out pattern, raw milk with poor quality such as more endospores, leakages in the product transfer pipeline, gasket leakages in the product pipeline, leaked aseptic chamber, upper cover of Tetra Fino, low consentration of H2O2, no tween80 added to H2O2, and leakages in cooling pipeling of aseptic air system in Tetra Fino may result such contamination pattern.
Pattern4/5: intermittent
•Probe leakage—seep into milk; •Operational fault—splicing PM; •Homoginizer fault resulted rough particles with spores; •Counter-Valve such as V78; •Gasket leakage or holding section leakages in UHT etc.
Pattern 6: consistency
Wrong operation such as selecting wrong sterilization program in UHT; Failure of equipment such as poor sealing, equipment components or equipment settings out of specification such as low sealing pressure, and poor quality milk with thermo resitant spores etc.
Pattern 7: Wave like
It is the combinition of intermittent pattern and consistent pattern.
Pattern 8: Random
Often it is caused by operational fault.
Pattern 9: Gradual increase
No bacteria multiplication, but reinfection increases. For example, the jaw system loses its pressure grdually. The white roller loses its pressure gradually.
Manufacturers and Processors requirements of ice cream, ice milk, ice cream mix and frozen youghurt
Requires 30 POINTS in the General category.Microbiological Quality of Ice Cream after HACCP Implementation: a Factory Case Study
Requires 30 POINTS in the General category.Movie - Avoid microbial infection or contamination in aseptic production
How ro remove microbial contamination in aseptic production is always a challenge. If the contamination is very little, the survivors will be less when processing. Once contaminated by microbes in the milk, the speed of microbes mulplification will be higher than the light. The visual coagulation or spoilage usually resulted by more than 100 million bacteria. Keeping a good habit in production is always a must such as clean hands routinely, clean floor routinely etc.
Keeping Cows Cool and Comfortable to Improve milk production of cow
When things get hot, we find ways to cool down. We fire up air conditioners, get wet, or get the air moving with fans. Our animals are no different. They feel the heat, and they want a break from it as well.
It’s a proven fact that when cows overheat, milk production goes down. It’s been proven time and again that severe heat stress in dairy cows can result in milk production loss of up to 25%. That is a quarter drop in overall milk production, so keeping your herd cool is imperative to keeping milk production up. There are a few different things that can be done to improve your herds comfort when the temperature starts to rise.
Have you ever been outside on a hot day and think…what I wouldn’t give for cold shower right now? When we sweat, we are using the moisture to draw the heat away from our skin. This same theory applies to cow cooling or misting systems. There are many out on the market today. At the farm where I worked, the misters were set up along the stalls, so that when the cows went to eat, they were “misted”. Be sure to use a system that uses water efficiently, but also uses enough water to penetrate the hide and reach the skin. It will then draw the heat away from the cow’s body, just like when we jump in a pool or take a cold shower. Our bodies are instantly cooled by the water drawing away the heat and this same theory applies to our animals.
Air movement is also critical. Stagnant air will feel hotter. Like the water drawing away the heat, so too can air movement. Fanning systems are a great way to help keep your herd cool and comfortable. There are also many systems out there, from your basic fans over the freestalls, to setting up a tunnel system where the air will actually be forced through the barn. When the air is moving, even in hot conditions, it can make a big difference in reducing heat stress in your herd.
Many systems combine these too main elements, water and air, using fans and sending a mist with the air to help reduce water waste while achieving the same or better results. It’s important to do your research first and find out what’s going to work best for your farm, and your herd.
Also, always have cool, clean water for your cows to drink. She needs to stay hydrated to help reduce heat stress, keeping her milk production high and at its best in hot weather.
What about calves?? They are no different when the heat kicks in. A few simple steps can be taken to help keep them cool and healthy as well. Besides methods mentioned above, there are a few small things you can do to ensure a calf’s comfort. Make sure they have clean, cool water to drink. Be sure that all pails are clean and sanitized after each use. If you need to handle the calves, consider changing the time of day when you do, opting for cooler parts of the day like early morning, or evening hours, and make sure shade is readily available. When a calf isn’t using all her energy to stay cool, she’ll be able to use that energy to grow and stay healthy.
With a few simple techniques your herd can stay cool and comfortable, and reducing heat stress will help keep milk production high even during the hottest times of year!
By Susan Glenzinski 07.2012
Israeli Dairy School
Operation and quality management
Introduction
To help you to start a toy manufacturing company , we strongly believe that Operation management and quality management is a very important factor, and could not be neglected in your management system. In order to prove this point, we will set out the overall impact as the beginning.
With the rise of the service sector, the concept of the further expansion of production, and gradually to accommodate the non-manufacturing service sector, including not only the manufacture of tangible products, but also include intangible services. The implementation of effective operations management is increasingly. Faced with such pressure, quality management become indispensable.
So firstly we should understand that the goal of the Quality Management program(QMP) is to establish leadership structure throughout the path of quality workflow that enables merry toy manufacturing company to provide high quality services. We are designed to promote quality and child’s entertainment and safety and also operations efficiency through process strategy,quality management, inventory management and supply chain management
Quality management
Business profile:
Merry is a toy manufacturing company. It deals with the production and sales of various toys. As we all know, children related consumption is a good gold digging point, thus it’s certain that many businesses go for it. Toy industry is one of the competitive battlefields.
There are mainly three components of quality management: quality control, quality assurance and quality improvement. Most of us would take the simple view that quality management is just concerned with product quality. In fact, that’s not complete. Quality management also deals with the control of manufacturing process. For the company whose dominant business is the manufacturing and selling of products, like Merry, quality management is especially important. High-quality product is the most basic thing your company should focus on to achieve an outstanding performance.
When it comes to quality management, the most classic case could be Six Sigma Methodology. Sigma is the standard deviation used in statistics. Six Sigma is a management approach near perfection, demanding that there must be less than 3.4 defective products or processes per million units. A number of world-class international corporations, like Motorola, Citigroup, Ford, Kodak and General Electric, once employed Six Sigma to keep their leading position in industry. The widely used management methodology characterizes in the following aspects:
- It highlights the customer demand and always treats it as the main breakthrough point.
- It emphasizes the measurement of performance and processes. Based on these, the company comes up with more challenging goals.
- It provides specific performance improving methods for specific purposes and fields.
- It owns a group of trained professionals in its implementation to guarantee the effect.
- It defines the standard and measurement of success as well as the rewarding methods to employees participating in the accomplishment.
- It breaks the barriers between different departments.
All of us know Jack Welch and General Electronic. In the mid and later years of 1990’s, Jack Welch became a loyal seeker of Six Sigma Management. This led to GE’s great success and the whole world’s eyes.
Actually, quality management has many classic theories besides Six Sigma, like PDCA Cycle (Plan-Do-Check-Action) and Total Quality Management. They have been raised and being used universally in today’s society. Those successful stories can give you some experience on the quality management. In the daily operation of your toy company, you can borrow and use it.
Process strategy
Business profile:
Merry is a toy manufacturing company. It deals with the production and sales of various toys. As we all know, children related consumption is a good gold digging point, thus it’s certain that many businesses go for it. Toy industry is one of the competitive battlefields.
To obtain an advantage over the rivals, process strategy and supply chain management are vital.
Process strategy is to figure out the way of doing right things to improve the productivity or efficiency. After recognition of a better process, the existing process can be optimized to achieve a much more satisfying performance. Let’s take an automobile manufacturing factory as an example. With rapid development, its scale and labour had increased a lot yet no change in management system. So a bottleneck occurred. Under the guidance of experts, the company began to realize the problems and optimize its process, including standardization of process instead of process mess among different departments, process function reinforcement instead of useless form, process supervision instead of ignorance of the implementation. The adoption of these process strategies did give much vigour to the company: the production had been soaring; what’s more, the employees had been more disciplined and organized. This example tells how the process strategy can help us make a breakthrough and yield twice the result with half the effort. Besides those measures, process strategy also includes process restructuring, process cutting and process control etc. In reality, most manufacturers neglect the importance of process optimization. They put all their emphasis on the equipment or technology. I hope your toy factory will draw a lesson from it. After all for a manufacturing factory, production is the top priority needed to be dealt with and is a critical competitive point. You will benefit a lot to take process strategy into consideration in the production of toys.
Supply chain management
According to Tom McGuffog, Supply Chain Management (SCM) is "Maximizing added value and reducing total cost across the entire trading process through focusing on speed and certainty of response to the market." Supply chain is so complicated a system that it needs efficient coordination and management. Huge invisible profits are hidden between every two chains. The purpose of SCM is to squeeze out those profits continuously. To understand this more vividly, I’d like to employ the most classic case Wal-Mart. As the global number one retail business, Wal-Mart’s SCM consists of four parts:
Customer demand management: All businesses know the motto “customer is God”. Only stick to our commitments to customers can we make the most profits. High quality products and high standard services are the basic things we should do.
Supplier and partner management: Wal-Mart has established strategic relationship with P&G. It provides P&G with plenty of room to demonstrate itself freely; it uses P&G’s fame to popularize itself. They are digging potential mutual benefits instead of grabbing the existing cake.
Inner-company and between-company logistics management: Wal-Mart established its own logistic system to guarantee most efficient and instant delivery. Marching with technology, the system has realized automation.
Information communication based on internet or intranet: Wal-Mart is the first retail business to launch a communication satellite. With the help of the satellite, Wal-Mart makes delivery keep the same pace with sales.
Maybe you can learn from the success of Wal-Mart and stress the importance of SCM. The key point of SCM lies in close relationships and cooperation between upstream and downstream companies. For your toy factory, for example, you can consider carefully the best location of factory for the convenience of delivery to your downstream customers. Also, you can design a customer feedback system to understand the customer’s real demand and comment at the first time.
We can see both process strategy and supply chain management are scientific approaches to achieve the effect of Pareto Optimality.
Inventory Management
Inventory management, the planning and controlling of inventories in order to meet the organization, is an important concern for managers in all types of businesses. Inventories are important to all types of organizations, their employees, and their supply chains. Inventories profoundly affect everyday operations because they must be counted, paid for, used in operations, used to satisfy customers, and managed. Nonetheless, companies realize that the availability of products is a key selling point in many markets and downright critical in many more.
Certainly, too much inventory on hand reduces profitability, and too little inventory on hand creates shortages in the supply chain and ultimately damages customer confidence. In this respect, Merry does very well. Despite the many changes that company goes through, the basic principles of inventory management remain the same. Some of the new approaches and techniques are wrapped in new terminology, but the underlying principles for accomplishing good inventory management has not changed. Its inventory management designed to meet the dictates of the marketplace and support the company's strategic plan.
The company of Merry uses J-I-T (just in time) and J-I-C (just in case) systems. The company provides the products what does customer want and when does customer want. Merry provides the products in time and in case. So, it decides that Merry should have a good inventory management. The inventory management system provides information to efficiently manage the flow of materials, effectively utilize people and equipment, coordinate internal activities, and communicate with customers. Inventory management does not make decisions or manage operations; they provide the information to managers who make more accurate and timely decisions to manage their operations.
So, many customers always like Merry! This good inventory management makes the company get more benefits and more money. Because of this Merry can be so development! It shows that how important the inventory management is!
Conclusion
Overall, good inventory management to meet the market domination and support the company's strategic plan. Good supply chain management to required the right product (Right Product) can be at the right time (Right time), in accordance with the correct amount of (Right Quantity), the right quality (Right Quality) and the correct state (Right Status) to the right place (Right Place), therefore, to increase the enterprise competitiveness. Good process strategy can be optimized to achieve a much more satisfying performance and improve the productivity or efficiency. Good quality management ensure product quality and increase core capability of enterprise. To bulit a good operation and quality management is a good foundation for business success.
References
- T McGuffog, N Wadsley, (1999) “The general principles of value chain management”.
- R Wilding, (1998), “The supply chain complexity triangle: uncertainty generation in the supply chain”.
P Trkman, MI Stemberger, (2005), “Information transfer in supply chain management”.
How to run an excellent service team
Service quality is promising to customer and expectation from customer affected by safety, reliability (No breakdown) and dependibility (Can solve problem) etc. which affects customer's reputation and product's consistency. So everything one service team does needs to be customer oriented. And customer oriented behaviors include competitive service benchmark or KPI, do right first time, keep continuous improvement. Especially when customer meets issues, the feedback must be quick and the result must be sustainable. To fulfil it, the root cause must be found and engage the customer in loop on a certain extent. The ultimate strategy is to satisfy customer by world class service performance. And the route is to have the qualified on-time service and solve problem quickly once it happens
If defects are detected from line, is it caused by machine itself or by the whole line?
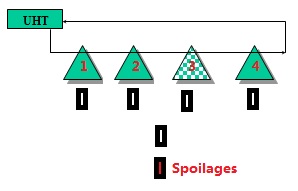
The production manager often meets the scenaria that series of machines are linked together forming one line to produce. And then we find the spoilages after being incubated. Do the soilages or unsterilities come from the indiviual machines or fillers such as 1, 2, 3, and 4. Or they come from UHT?
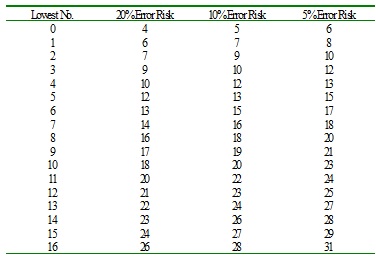
The significance table can give you the answer.
we need to take samples from the doubted filler and the reference. For example, we doubt the defects come from machine 3 and the other machines are normal. When taking samples from Filler 3, we also need to take samples from one reference machine such as 1, 2, or 4.
If the spoilages are found from machine 3 being 9, and 3 defects are found from filler 1, 2 and 4 at the same time. We can have the conclusion that machine 3 has its own problem besides the common problems from all of the mchines at the risk of 20%. If spoilages from filler 3 are 10 or 12, the rish will be 10% or 5% respectively.
We can query the counted unsterilities from different machines to compare. And often we use the risk being 10% to judge.
Innovation thinking
Q-questioning
O-observing
E-experimenting
N-networking
A-associating
Samples change
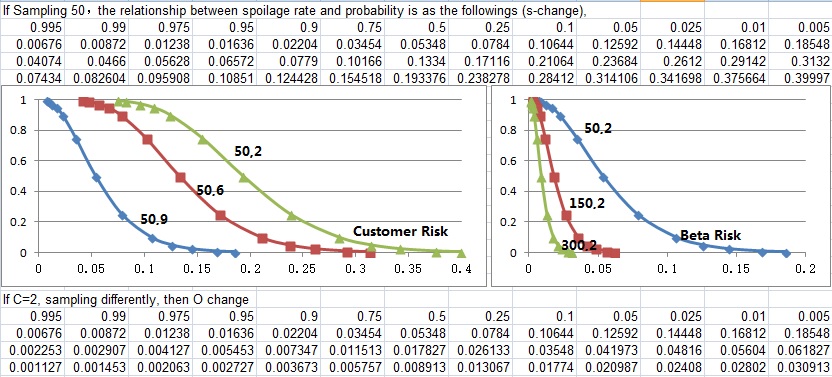
Samples change will affect alpha (manufacturer) risk, and beta (Customer) risk. O curve sampling change affects customer risk more dramatically than S curve sampling change. When detecting same spoilage rate, sampling more means less customer risk, but more manufacturer risk. S curve change is samples remains unchanged with more spoilages found. And O curve change is that samples increase with spoilages reamain unchanged.